Abstract
Background
Anthocyanins are common substances with many agro-food industrial applications. However, anthocyanins are generally considered to be found only in natural plants. Our previous study isolated and purified the fungus Aspergillus sydowii H-1, which can produce purple pigments during fermentation. To understand the characteristics of this strain, a transcriptomic and metabolomic comparative analysis was performed with A. sydowii H-1 from the second and eighth days of fermentation, which confer different pigment production.
Results
We found five anthocyanins with remarkably different production in A. sydowii H-1 on the eighth day of fermentation compared to the second day of fermentation. LC-MS/MS combined with other characteristics of anthocyanins suggested that the purple pigment contained anthocyanins. A total of 28 transcripts related to the anthocyanin biosynthesis pathway was identified in A. sydowii H-1, and almost all of the identified genes displayed high correlations with the metabolome. Among them, the chalcone synthase gene (CHS) and cinnamate-4-hydroxylase gene (C4H) were only found using the de novo assembly method. Interestingly, the best hits of these two genes belonged to plant species. Finally, we also identified 530 lncRNAs in our datasets, and among them, three lncRNAs targeted the genes related to anthocyanin biosynthesis via cis-regulation, which provided clues for understanding the underlying mechanism of anthocyanin production in fungi.
Conclusion
We first reported that anthocyanin can be produced in fungus, A. sydowii H-1. Totally, 31 candidate transcripts were identified involved in anthocyanin biosynthesis, in which CHS and C4H, known as the key genes in anthocyanin biosynthesis, were only found in strain H1, which indicated that these two genes may contribute to anthocyanins producing in H-1. This discovery expanded our knowledges of the biosynthesis of anthocyanins and provided a direction for the production of anthocyanin.
Similar content being viewed by others
Background
Anthocyanins are a class of flavonoids that have many agro-food industrial applications such as natural dyes [1]. More recent studies have shown that anthocyanins have potential preventive and/or therapeutic effects on human health, such as improving cardiovascular function and treating obesity [2, 3]. There are six common anthocyanidins: pelargonidin (Pg), peonidin (Pn), cyanidin (Cy), malvidin (Mv), petunidin (Pt) and delphinidin (Dp). Usually, people believe that the anthocyanins could only be derived from the secondary metabolism of plants.
The biosynthesis of anthocyanins in plants has been widely elucidated and well-understood. First, phenylalanine is converted into 4-coumaryl CoA. The conversion is regulated by phenylalanine lyase (PAL), cinnamate hydroxylase (C4H) and 4-coumaroyl CoA ligase (4CL). Second, dihydroflavonol is derived from 4-coumaryl CoA with the help of chalcone synthase (CHS), chalcone isomerase (CHI) and flavanone-3-hydroxylase (F3H). Then, dihydroflavonol is transformed into anthocyanins with the help of dihydroflavonol reductase (DFR) and leucoanthocyanidin dioxygenase (LDOX). After that, the glycosylation of anthocyanins is regulated by flavonoid glycosyltransferase (UGTs) [4, 5]. Finally, Pg and Mv are synthesized from Cy and Dp, respectively, with the help of O-methyltransferase (OMT). Among the genes involved in anthocyanin synthesis, C4H is one of the core genes of the phenylpropanoid pathway and mediates the synthesis of secondary metabolites such as anthocyanins [6] and artemisinin [7]. CHS links the phenylpropanoid pathway and the flavonoid pathway as well as plays an important role in the biosynthesis of anthocyanins [8].
In addition to functional genes, other researchers have reported that some regulatory genes played a pivotal role in controlling the synthesis of anthocyanins. Zhou H et al. found that R2R3-MYB can activate the promoters of proanthocyanin synthesis genes to regulate anthocyanin accumulation in peach flowers [9]. Tirumalai V et al. found that micro RNA (miRNA), miR828 and miR858, repressed anthocyanin accumulation though mediating VvMYB114 in grape [10]. Zheng T et al. found that HAT1 regulated anthocyanin accumulation via posttranslational regulation of the MYB-bHLH-WD40 (MBW) protein complex [11]. The findings suggested that we consider the roles of those regulatory genes, including miRNA, lncRNA play in regulating in the biosynthesis of anthocyanins.
As the understanding of metabolomics continues to deepen, several metabolites that were used to be only produced in plants have been produced in microorganisms. For example, betalain in Penicillium novae-zelandiae [12]; lawsone, an orange-red pigment, in Gibberella moniliformis [13]; and Taxol and related taxanes in Aspergillus niger [14]. Regarding to the production of anthocyanins from microorganisms, there is no clear confirmation before even though some of the activities of anthocyanin-related genes such as PAL, C4H and 4CL have been detected during Alternaria sp. MG1 fermentation [15].
Aspergillus sydowii, first named in 1926 by Charles Thom and Margaret Brooks Church [16], was reported as a pathogen of gorgonian corals [17, 18] and found in different habitats where it survives as a soil decomposing saprotroph [19, 20]. Meanwhile A. sydowii has been widely studied for its ability to biodegrade agrochemicals and contaminants [21,22,23]. Moreover, some novel secondary metabolites, such as antidiabetic and anti-inflammatory sesquiterpenoids [24], sesquiterpene and xanthone [25], 2-hydroxy-6-formyl-vertixanthone, 12-O-acetyl-sydowinin A [26], and indole alkaloids [27], were found and identified in A. sydowii. These observations demonstrate the capability and complexity of the fungi strain A. sydowii in orchestrating the biosynthetic routes of their secondary metabolites.
As reported previously we have successfully isolated and identified a fungi strain H-1 from humus that cultivated bacterial wilt-affected ginger in Chengdu, China, at 2016 [28]. We proved that H-1 belonged to the fungi strain Aspergillus sydowii by morphology and phylogeny methods (ITS accession number: MN263259, beta-tubulin accession number: MH426599.1). During the fermentation, we observed a purple pigment has been produced by Aspergillus sydowii H-1. In this study, we analyzed and confirmed that the purple components are anthocyanins, explored the anthocyanin synthesis pathway of A. sydowii H-1, and investigated the evolutionary relationship between anthocyanin synthesis pathways in fungi and the corresponding pathways in plants. Finally, we also found three regulatory genes were actively involved in the anthocyanin biosynthesis pathway. Our studies firstly discovered that anthocyanin could be produced in the fungi, which will provide new strategies and perspectives for the production of anthocyanins.
Methods
Extraction and purification of the purple pigments from Aspergillus sydowii H-1
Aspergillus sydowii H-1 was cultured on Czapek Dox agar medium. Spore suspensions were prepared from 7-day-old culture slants by adding an adequate amount of sterile distilled water. The spore number was 1.6 × 106 cells/mL, which was inoculated into 200 mL of seed culture (Chest’s medium) at 28 °C 180 rpm/min for 60 h. Then, 10 mL of the above mycelial suspension (5% v/v) was inoculated into 200 mL of fermentation medium. The 1 L fermentation medium was composed of 5 g of glucose, 3 g of peptone, 0.5 g of yeast extract, 1 g of KH2PO4, and 1 g of NaCl. On the 2th (G2) and 8th (G8) day of fermentation, the broth was collected and purified by DM130 macroporous resin. The elution flow rate was 1.5 mL/min and 70% ethanol at a flow rate of 1 mL/min. Then, both the G2 and G8 fermentation broth treated with DM130 macroporous resin were freeze-dried into powder and stored at 4 °C.
Identifying the purple pigments and determining the biochemical properties during fermentation
Three biochemical properties (fungal biomass, pigment yield and the content of reducing sugar) were monitored from the first day to the 11th day, and all experiments were repeated three times. The dinitrosalicylic acid (DNS) method was used for the quantitative analysis of reducing sugar [29]. The biomass of H-1 was determined by gravimetric analysis after filtering the cell samples through a pre-weighed nylon filter fabric mesh (74 μm porosity) and dried to constant weight at 60 °C. The purple pigment was extracted from the liquid medium through a water-soluble filter of 0.45 μm pore size (Jing Teng, China) and centrifuged at 12,000 rpm for 10 min. The characteristic absorption peak of the purple fermentation broth was scanned at 400~800 nm with a spectrophotometer (Thermo, U.S.A). The content of the purple pigment (extracellular) was quantified indirectly by simply measuring the optical density (OD) at 520 nm, which was the maximum absorption wavelength for the pigment, using a spectrophotometer. Raw data from a time course of biomass, sugar consumption and crude pigment content are shown in Additional file 1: Table S1.
The chemical group of the purple pigment contained was identified by Fourier transform infrared spectroscopy (FTIR). FTIR spectra determination was acquired using Nexus 6700 (Thermo, USA). The above G8 purple lyophilized powder was thoroughly mixed with KBr and pelletized. The resolution of the obtained spectrum was 0.09 cm− 1, and the range was 4000–400 cm− 1, as described in C.S. Pappas et al. [30, 31].
Metabolome analysis of Aspergillus sydowii H-1 fermentation broth
The lyophilized powder from G2 and the G8 with three independent biological replicates was prepared for downstream analysis. First, 0.1 g of the G2 and G8 powder was extracted overnight at 4 °C with 1.0 mL of 70% methanol aqueous solution and centrifuged at 10,000 rpm/min for 10 min. Following centrifugation at 10,000 g for 10 min, the extracts were absorbed (CNWBOND Carbon-GCB SPE Cartridge, 250 mg, 3 ml; ANPEL, Shanghai, China) and filtered (SCAA-104, 0.22 μm pore size; ANPEL, Shanghai, China) before LC-MS analysis. A quality control sample was prepared by equally blending all samples. During the assay, a quality control sample was run every 10 injections to monitor the stability of the analytical conditions.
The analytical parameters in the LC-ESI-MS/MS system were as follows: HPLC column, Waters ACQUITY UPLC HSS T3 C18 (1.8 μm, 2.1 mm*100 mm); solvent system, water (0.04% acetic acid): acetonitrile (0.04% acetic acid). A gradient elution was performed as follows: 100:0 V/V at 0 min, 5:95 V/V at 11.0 min, 5:95 V/V at 12.0 min, 95:5 V/V at 12.1 min, 95:5 V/V at 15.0 min; flow rate, 0.40 ml/min; temperature, 40 °C; injection volume, 5 μL.
Metabolites were identified on a 6500 QTRAP system (Applied Biosystems, Foster City, CA, USA) equipped with an electrospray source. The ESI source operation parameters were as follows: ion source, turbo spray; source temperature, 550 °C; ion spray voltage (IS), 5500 V; ion source gas I (GSI), gas II (GSII), and curtain gas (CUR) were set at 55, 60, and 25.0 psi, respectively. Instrument tuning and mass calibration were performed with 10 and 100 μmol/L polypropylene glycol solutions in triple quadrupole (QQQ) and LIT modes, respectively.
Qualitative analysis was performed according to the method reported previously [32]. Orthogonal projections to latent structures-discriminate analysis (OPLS-DA) was performed on the identified metabolites. Variable importance in projection (VIP) ≥ 1, fold change ≥2 and P-value ≤0.5 were used as the threshold of significantly different metabolites.
RNA extraction, cDNA library preparation, and RNA sequencing
RNA was isolated, and cDNA libraries were constructed on the second fermentation day and the eighth fermentation day (three replicates for each time point) according to the Illumina HiSeq X-Ten (Illumina, San Diego, CA) RNA library protocol. Library sequencing was performed on a HiSeq X-Ten (Illumina) platform to obtain 150 bp paired-end reads. The raw sequencing reads were submitted to the National Center for Biotechnology Information (NCBI) (BioProject: PRJNA542911).
RNA-Seq analysis pipeline
Low quality reads were trimmed by Trimmomatic (version 0.36) [33]. The reads that mapped to the known transfer RNAs (tRNAs) and ribosomal RNAs (rRNAs) were removed by searching the Rfam database via Bowtie2 2.3.2 [34]. Then, the trimmed and rRNA-free reads were mapped to Aspergillus sydowii CBS 593.65 [35] with Hisat2 (version 2.1.0) [36], and transcripts were assembled with StringTie (version 1.3.3b) [37] by a reference-guided method with default parameters. In order to discover more sequences that are not present in the reference genome, that is, transcripts unique to A. sydowii H1, Trinity version 2.8.4 [38] was used to assemble transcripts by the de novo method with the default parameters. De novo assembled transcripts shorter than 300 bp were discarded, and the longest transcript in each cluster (gene) was selected as the representative of the unigene. By comparing the reference-guided sequences with the de novo-assembled unigenes by blastn, the sequences that aligned to reference-guided sequences were removed. The remaining unigenes appeared as the de novo assembly results. The union of the genes obtained by the two methods was used as the final genes in A. sydowii H-1. The gene expression levels were calculated and normalized via the expectation maximization method with RSEM version 1.2.31 [39].
In order to obtain the functional annotation of the de novo-assembled unigenes, the coding sequences (CDSs) and the translated protein sequences of the unigenes were predicted with TransDecoder version r20140704 (http://transdecoder.github.io/, accessed 26 Sept. 2018). Then, proteins were functionally annotated by blastp (Camacho et al., 2009) based on queries of functional databases, including the SwissProt database, NCBI nonredundant database and RefSeq database. Pathway annotation of the Kyoto Encyclopedia of Genes and Genomes (KEGG) terms was performed using KOBAS version 3.0 [40], and protein domains were annotated using IterProScan5 version 2.0 (https://github.com/ebi-pf-team/interproscan) against with the Pfam database. Differentially expressed genes (DEGs) between G2 and G8 were analyzed using the DESeq2 package [41]. P-value ≤ 0.05 and the absolute value of fold change ≥2 were set as the threshold for identifying significantly differentially expressed genes.
LncRNA analysis pipeline
First, the NONCODE database [42] was used to characterize the annotated lncRNAs in A. sydowii H-1 from the assembled transcripts, but none of the transcripts matched the known lncRNAs. Then, to identify novel lncRNAs, we followed the steps below to filter novel lncRNAs from the newly assembled transcripts. Multiple-exon transcripts were considered to be expressed if they had a TPM (transcripts per million) greater than 0.5. For single-exon transcripts, more rigorously, the TPM was greater than 2. Those foregone coding genes or transcripts with sizes less than 200 nt were filtered out. Last but not least, lncRNA candidates were identified by CPC2 (version 0.1) [43], CNCI (version 3.0, [44], PLEK (version 1.2) [45] and LGC (version 1.0) [46]. Candidate transcripts predicted to have noncoding potential by two or more programs and did not contain any known structural domains were considered the lncRNAs in A. sydowii H-1.
To reveal the potential function of the lncRNAs, their target genes were predicted for both trans- and cis-acting functions. Cis-acting, refers to the action of lncRNAs on neighboring target genes. In this study, coding genes ranging from 100 kb upstream and downstream of lncRNAs were searched for cis-acting target genes. The trans role refers to the influence of lncRNAs on other genes at the expression level. RNAplex [47] and LncTar [48] software were used to predict lncRNA target genes that were trans-acting. Finally, the Pearson correlation coefficient between lncRNAs and their target genes was calculated by R language. High confidence pairs (|cor| ≥ 0.7 and P-value≤0.5) seemed to be the most likely interaction between the lncRNA and its target gene.
Phylogenetic trees with 2-oxoglutarate-dependent oxygenases (2-ODD) families
The protein sequences from the 2-ODD family, including the candidate transcripts and known anthocyanin-related genes, were aligned using MUSCLE version 3.8.31 [49] with the default parameters, and the corresponding CDSs were back-translated from the corresponding protein sequences. The conserved CDSs were extracted with the Gblocks method [50]. The bootstrap consensus of the phylogenetic tree was inferred from 100 replicates. Maximum likelihood trees were compiled with RAxML version 8.2.7 software [51] and edited with iTOL (https://itol.embl.de).
Real-time quantitative PCR (RT-qPCR) validation
Total RNA was extracted from 100 mg of fungal mycelia using TRIzol reagent (Invitrogen, Carlsbad, CA). Reverse transcription was performed using the PrimeScript™RT reagent Kit with gDNA Eraser (TaKaRa). Nine anthocyanin-related genes were selected for RT-qPCR, and the specific RT-qPCR primers were designed with Primer Premier 5 software (Additional file 5: Table S5). Primers for RT-qPCR were synthesized by the Chengdu Qingke Zi Xi Biotechnology Company (Chengdu, China).
With the relative quantitative method, each quantitative reaction was performed in a reaction mixture with a total volume of 25 μL, including 12.5 μL of 2× SYBR Premix Ex Taq TM II (TaKaRa), 2 μL of diluted cDNA template, 1 μL of each primer (10 μM) and 8.5 μL DNase-free water. The amplification was predenatured at 95 °C for 30 s, denatured at 95 °C for 40 cycles for 5 s, and annealed and extended at 60 °C for 34 s. Three technical replicates were tested for each gene, β-tubulin was used as an internal reference gene, and the 2 − ΔΔCT method was used to calculate the relative expression of the genes. All data displays and statistical analyses were performed using GraphPad Prism 5. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, are given in the figure legends.
Results
The characterization of fermentation and the preliminary identification of anthocyanins
The fermentation characteristics of A. sydowii H-1 were monitored (Fig. 1d). During the day 1 and day 2, A. sydowii H-1 was in a growth delay period with slow sugar consumption rate. At this period no pigments were observed. During days 3–7, the fungi were in the logarithmic growth phase. The mycelium grew rapidly with rapid glucose consumption. The UV-visible absorption spectroscopy showed that the characteristic absorption peak at 520 nm gradually increased, indirectly indicating the accumulation of the purple pigments. We observed a maximum pigment absorbance of the fermentation broth at the 8th day of fermentation. The fungi were in a stable phase with slow growth. The weight of the cells decreased in the last three days of culture, and the hyphae began to disintegrate because of autolysis. At the same time, the mycelium no longer produced purple pigments.
The characteristic of the purple pigment produced by H-1. (A) Color change of the A. sydowii H-1 fermentation broth from day 1 to day 11. (B) Characteristic absorption peak of purple determined with a spectrophotometer. (C) Identification functional group of the purple substance by FTIR. (D) Time course of biomass, sugar consumption, and crude purple pigment production. Abs, absorbance value; OD, optical density
According to the growth curve of A. sydowii H-1 and the rough production of pigments, the two key time points of A. sydowii H-1 fermentation were the 2nd day, the pregrowth period with no significant accumulation of pigments, and the 8th day, the stable period of the cells with the highest accumulation of pigments. Therefore, we selected the fermentation broth and cells on the second (G2) and eighth (G8) days for subsequent metabolome and transcriptome analysis.
With the increasing of fermentation time, the concentration of pigments was gradually increased (Fig. 1a). The absorption spectroscopy of the fermentation broth (Fig. 1b) showed that the fermentation broth had a maximum absorbance at 520 nm, which was consistent with the absorption peak of anthocyanins [52]. We further confirmed the chemical structure by the FTIR spectra of freeze-dried powder derived from the purple fermentation liquid (Fig. 1c). The stretching band at 3394.10 cm− 1 corresponds to the OH vibration of the hydroxyl group [53]. The major peaks observed for chitosan were 1647.07 cm− 1 (amide I band) [54], and peaks at approximately 800–1150 cm− 1 are characteristic of polysaccharides assigned to the C–O valence vibrations and C–O–C stretching vibrations of carbohydrates, including fructose, glucose and glucomannan. Peaks between 1133 and 1457 cm− 1 correspond to anthocyanins [55].
We have proved that the absorption spectroscopy and the FTIR assays of the purple pigment were consistent with the characteristics of anthocyanins. Although the consistency could only provide a preliminary structure confirmation of the purple pigments [56,57,58], we were able to apply metabolomics analysis to further validate the composition of the purple pigment produced by A. sydowii H-1 (see below).
Widely targeted flavonoid metabolomics
To further determine the composition of the purple pigment produced by A. sydowii H-1, we defined two time-points during the fermentation process. One is G2, the pre-growth period at day 2 and the other is G8, the stable period at day 8. Since anthocyanins is a type of flavonoid, we performed LC-MS/MS analyses to analyze flavonoid metabolite. As a result, we identified 85 flavones including seventeen flavonols, ten isoflavones, eight flavanones, seven anthocyanins, six polyphenols and thirty-eight other flavones (Additional file 2: Table S2). An OPLS-DA model with R2Y(cum) = 0.98 and Q2Y(cum) = 0.99 (Additional file 6: Figure S1), was constructed and was able to distinguish the G8 samples from the G2 samples. The results of the permutation test of the OPLS-DA model were R2Y(cum) = 0.35 and Q2Y(cum) = − 1.25 (Additional file 6: Figure S2). The low values of the Q intercept indicated the robustness of our models and thus showed a low risk of overfitting, indicating that the model was reliable.
The metabolites distinguishing the two incubation periods were listed in a heatmap; thirty-nine metabolites were significantly different (VIP ≥ 1, fold change ≥2 and Q-value≤0.05) between G2 and G8 (Fig. 2a) (Additional file 2: Table S2), including five anthocyanins (Fig. 2b): peonidin o-malonylhexoside (peonidin-Mh), cyanidin 3-O-glucoside (kuromanin), cyanidin, malvidin 3-O-galactoside (malvidin-3Ga) and malvidin 3-O-glucoside (oenin). Among those anthocyanins oenin and malvidin-3G were more abundant than the others. Compared the concentration of oenin and malvidin-3G at G8 to G2, there are 8267- and 6147-fold increase, respectively. Because these kinds of anthocyanins have been reported in berries (Lonicera caerulea, Rubus fruticosus, Ribes nigrum and Morus alba), cereals (Zea mays) and vegetables (Brassica oleracea, Dioscorea alata, Daucus carota and Asparagus officinalis) [59,60,61], the LC-MS/MS analysis results have verified that the purple pigment yielded by A. sydowii H-1 is anthocyanins.
An overview of the changes in flavonoid compounds in A. sydowii H-1 in different fermentation stages. (A) Differences in the primary metabolite profiles in different fermentation stages; the heatmap color indicates the abundance of each metabolite in different fermentation stages. (B) The significant differentially content change in anthocyanin concentration relative to the second day (G2). (G2, the fermentation broth of the second day; G8, the fermentation broth of the eighth day; malvidin-3Ga, malvidin 3-O-galactoside; peonidin-Mh, peonidin O-malonylhexoside)
Transcriptome sequencing, transcript construction and the analysis of differentially expressed genes
After confirming the composition of the metabolites, RNA-Seq was used to construct the transcripts of A. sydowii H-1 in both the second (G2) and eighth (G8) days with three biological replicates. After the removal of adaptor-contaminated, low-quality and rRNA reads, the clean reads from RNA-seq were aligned to Aspergillus sydowii CBS 593.65 [35] by Hisat2 version 2.0.4 [36], and the mapped ratio ranged from 78.47 to 91.04%. The mean GC content and Q30 were 53.41 and 94.16%, respectively (Table 1). A high Q30 value indicates that the sequencing data are authentic. Assembly was performed using the reference-guide and de novo method to obtain transcripts as complete as possible (see method). In total, 13,045 gene loci consisting of 15,161 transcripts, including 14,376 reference-guide-derived transcripts and 785 de novo-derived transcripts, were obtained. The transcript levels were estimated with RSEM 1.2.31 [39] software. Pearson correlation analysis between samples performed on the expression matrices of the genes (Additional file 4: Figure S4) showed that there was a difference trend between T5 and other biological repetitions of the G8 period. This may be due to the different growth conditions of fungi in the same fermentation stage. Therefore, the subsequent expression-related analysis will not include T5. Gene differential expression analysis identified 5243 differentially expressed genes (DEGs) (|fold change| ≥2 and P-value ≤ 0.05) (Additional file 3: Table S3). To verify the accuracy of RNA-seq, we selected some genes for RT-qPCR. The trend in the expression levels of all selected genes was consistent with the RNA-seq data, which proved that our transcriptome data were authentic. (Fig. 6, Additional file 4: Table S4).
Identification of anthocyanin-related genes
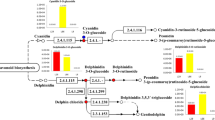
Although the synthesis pathway of anthocyanins in plants has been studied in details, the anthocyanin-related genes in fungi have not yet been fully explored. In this study, we identified a total of 28 anthocyanin-related genes (Table 2, Fig. 3a), and developed the pathway diagram referring to Guy Polturak et al. [62]. According to Pelletier’s study [63], we divided these genes into early biosynthetic genes (EBGs) and late biosynthetic genes (LBGs). Among these genes, 4CL, which is the key to the general phenylpropanoid pathway and participates in monolignol biosynthesis through the production of p-coumaroyl-CoA [64], had the largest number of paralogs genes. It is worth noting that C4H and CHS were only found in our own de novo assembled unigenes. Among them, cinnamate 4-hydroxylase (C4H, EC 1.14.13.11) is the second enzyme of the phenylpropanoid pathway and a member of the cytochrome P450 family. Chalcone synthase (CHS, EC 2.3.1.74) is a key enzyme that catalyzes the first committed step in the flavonoid biosynthetic pathway. Moreover, the best hits of C4H and CHS all blast against plant species genes with very high identity and query coverage (over 95%) (Additional file 5: Table S5). Therefore, similar to the biosynthetic mechanisms in the plant these two genes may contribute importantly to the production of anthocyanins in A. sydowii H-1.
Statistics of gene functions related to anthocyanin metabolism in A. sydowii H-1. (A) Putative anthocyanin synthesis pathway found in A. sydowii H-1. Flavonols can be further modified by glycosylation to produce flavonol glycosides. Anthocyanin-related genes can be classified into two major pathways: the phenylpropanoid biosynthesis pathway and the flavonol biosynthesis pathway. The anthocyanin-related compounds identified in A. sydowii H-1 are enclosed in purple boxes. CHS and C4H, which were only found in H-1, are in red circles, and other genes found in the reference genome are in pink circles. Three lncRNAs, which were found in this study, are in light blue boxes (B) Heat map of the expression of genes involved in the anthocyanin synthesis pathway in A. sydowii H-1. The asterisk indicates a transcript with a significant difference in expression between the two fermentation stages (|fold change| ≥2, P-value≤0.05). Malvidin-3Ga, malvidin 3-O-galactoside; peonidin-Mh, peonidin O-malonylhexoside
Except for CHS and C4H, all other genes were found to be the best hits with other fungal species genes. To explore the evolutionary relationship of the remaining anthocyanin-related genes between fungi and plants, the 2-oxoglutarate-dependent oxygenases (2-ODD) family, which includes anthocyanin biosynthesis-related genes, such as leucocyanidin oxygenase gene (LDOX), flavanol synthase gene (FLS) and flavanone 3-dioxygenase gene (F3H), was used for constructing a phylogenetic tree with known homologous sequences in other fungi and plants (Fig. 4). The results showed that all three types of genes were clearly separated according to fungi or plants rather than the type of genes. This separation indicated that the anthocyanin synthesis pathway genes may have evolved separately in plants and fungi. Furthermore, these genes in plants can be clearly divided into different branches according to different gene types but difficult to separate clearly in fungi. This manifestation revealed that these three types of genes may differentiate earlier in plants than in fungi.
Analysis of the lncRNA genes related to anthocyanins
There are several studies have found that lncRNAs performed a variety of functions in different important biological processes [65,66,67]. However, the role of lncRNAs in regulating anthocyanin synthesis has not been adequately studied. Hence, in this study we examined a total of 530 lncRNAs that were identified in A. sydowii H-1. 146 of the lncRNAs showed significantly differential expression between the G2 and G8 samples (|fold change| ≥ 2, P-value≤0.05). Among them, 144 lncRNAs regulated target genes by either cis-acting or trans-acting mechanism (Fig. 5a). We followed two steps to filter lncRNAs that may interact with anthocyanin synthesis-related genes: 1) The target genes of the lncRNAs must be an anthocyanin synthesis-related gene (|cor| ≥ 0.7, P-value ≤0.05) and 2) There should be a high correlation (|cor| ≥ 0.7, P-value≤0.05) between the lncRNAs and at least one kind of anthocyanin compound identified by LC-MS/MS. Finally, three lncRNAs appeared to regulate two genes involved in anthocyanin synthesis. One functional gene, ASPSYDRAFT_91437, and three lncRNA genes, MSTRG.7153.1, MSTRG.3144.1, and MSTRG.3145.1, were differentially expressed in G2 and G8 (Fig. 5b, Table 3). The correlation between the function and lncRNA genes involved in anthocyanin synthesis and major flavonoid compounds is presented as a correlogram in Fig. 5c. Interestingly, the highest and most significant positive correlation was found between 4CL and flavonoids. These results suggest that 4CL may act as a gatekeeper in the flavonoid biosynthesis pathway and play an important role in regulating the biosynthesis of anthocyanins in A. sydowii H-1.
Genes and lncRNAs related to the anthocyanin synthesis pathway. (A) Venn diagrams illustrating the number of differentially expressed cis-acting and trans-acting lncRNAs. (B) Positional relationship between the lncRNAs (red dots) and the corresponding target genes (purple triangles). The direction of the triangles represents the positive and negative strand in the genome, and the size of the triangle represents the relative size of the locus of the gene. (C) Bubble plot showing the correlation between anthocyanin compounds and related structural genes or lncRNAs. Only those with a P-value lower than 0.05 are shown. Cis, cis-acting; trans, trans-acting; malvidin-3Ga, malvidin 3-O-galactoside; peonidin-Mh, peonidin O-malonylhexoside
Discussion
Anthocyanins are one of the most widespread families of natural pigments in plants and are a class of compounds belonging to flavonoids [68]. Here, we reported a fungus, Aspergillus sydowii H-1, could produce anthocyanins under specific fermentation and analyzed the metabolome and transcriptome of these anthocyanins. This is the first time to report the formation of anthocyanins in a fungus as far as we know. This discovery has significant implications for the industrial production of anthocyanins and deepened our understanding of anthocyanins.
The highest content of the six identified anthocyanins was oenin and malvidin-3G, which belong to the malvidin ramification. This result was similar to the anthocyanins components that were reported in blueberries [69], red rice [70] and grapes [71]. Malvidin has been shown to protect against cardiovascular disease [72], and attenuate H2O2-induced oxidative stress [73] and inflammation [74], demonstrating that this strain has great potential for medical application. Except for malvidin, other content of anthocyanin components in the purple pigments were not exactly the same as that of anthocyanin components reported in above-mentioned plants. This finding suggests that anthocyanin synthesis in fungi is regulated by complex networks different from these in the plants. The specific mechanism in fungi still needs to be further explored.
After trimming, mapping and assembling the transcriptome data, we obtained a total of 15,161 transcripts that included the 28 transcripts involved in anthocyanin synthesis. Interestingly, the expression of LBGs increased after 8 days, while all of EBGs decreased (Fig. 3, 5, 6b, b). The 8th day is the time when anthocyanin accumulated the most in the fermentation broth with declining cumulative rate, and the reduced expression of the relevant genes. These activities increased nutrient consumption and decreased biomass (Fig. 1d). We hypothesized that EBGs in A. sydowii H-1 may play a more important role in the yield of anthocyanins than previously imagined.
C4H and CHS are vital functional genes in existing studies regarding the process of anthocyanin genes [6, 8, 75]. However, CHS and C4H could be assembled only by the de novo method, which suggests that the reference genome, CBS 593.65 [35], may not contain these two genes. To rule out errors in gene structural identification, we further mapped the protein sequences of these two genes to the genome using GeneWise [76] with default parameters. However, GeneWise proved the same results, thus negating the possibility that an error occurred in the gene structure annotation. This evidence proves that CHS and C4H are absent from the reference genome; that is to say, these genes were found in our strain and not in the CBS strain.
Except for the discussion of specificity, we also found these two genes, although only the core domain was assembled, in plant species. The CHS_1, CHS_2 and C4H sequences were searched against the NCBI nonredundant database by blastn, which determined that CHS_2 and C4H had the highest identity with Fagopyrum esculentum, Fagopyrum tataricum and Acacia mangium (Additional file 5: Table S5). More interestingly, the alignment method we selected was blastn (nucl-nucl) instead of blastp (prot-prot) or blastx (nucl-prot); such high homology allows us to propose that A. sydowii H-1 may have obtained these two genes through plant-fungal horizontal gene transfer (HGT). There are numerous studies suggesting that HGT is an important mechanism in eukaryotic genome evolution, particularly in unicellular organisms [77]. Though phylogenetic inference, Giovanni Emiliani et al. found that the ancestor of land plants acquired a PAL via HGT during symbioses with fungi that protect against ultraviolet radiation and capture pigment [78]. Thomas A. Richards et al. defined 14 candidate plant-fungi HGT events by phylogenomic analysis, nine of which showed an infrequent pattern of HGT between plants and fungi, and some kind of genes were involved in transport of the sugar L-fucose and other functions [79]. Meng Li et al. identified 19 fungal genes that had been transferred between fungi and bacteria/plants, which suggests that HGT might have played a role in the evolution and symbiotic adaptation of this arbuscular mycorrhizal fungus [80]. However, due to the incomplete assembly of the transcripts, we will continue to identify the origin of these two genes with the support from complete genomic information.
Among the extensive cortège of plant-associated microorganisms, striking examples of soil microorganisms are mutualistic fungi that have successfully coevolved with their hosts since plants adapted to terrestrial ecosystems [81]. In natural ecosystems, the antagonistic interaction between plants and their pathogens drives a coevolutionary dynamic in which pathogens evolve to avoid plant defense systems and plants evolve to recognize fungal pathogens [82]. Such as Zizhang Wang et al. found a new system, mediating PAMP- and effector-triggered immune responses between peanut and Aspergillus flavus, belongs to a three- grade coevolution of plant−pathogen interaction [83]. R. Hung hypothesis that Aspergillus spp. may help plant obtain nutrients beyond its normal capacity and induce systemic changes through extracellular exudates [84]. Asaf Levy et al. found that plant-associated bacteria have ostensibly evolved genes, some of which potentially mimic plant domains or are shared with plant-associated fungi and oomycetes that enable them to adapt to plant environments [85]. Anthocyanins as secondary metabolites protect plant tissues/organs against many abiotic stressors by different mechanisms, such as sunscreen, antioxidant and metal-chelating [86]. Whether the biological meaning of anthocyanins produced in Aspergillus sydowii H-1 is a convergent evolution with plants to cope with stress or to mimic the structural domain or function of plant genes to evade plant immune monitoring as mentioned before requires further research.
We further studied regulatory genes, such as long noncoding RNA, which may participate in the anthocyanin synthesis pathway. We predicted that three lncRNAs were involved in the regulation of the anthocyanin-related genes DFR and UGT. Thus far, it has been reported that these two genes can be regulated in other ways. DFR is a key enzyme that regulates metabolic processes involved in anthocyanin synthesis. It can catalyze dihydroflavonol to produce leucocyanidin. Blue J. Plunkett et al. discovered that the transcription factor MYBA is able to transactivate the DFR promoter in plants without dependence on a coinfiltrated bHLH cofactor [87]. UGT, the final enzyme in anthocyanin biosynthesis, catalyzes glucosyl transfer from UDP-glucose to the 3-hydroxyl group to form stable cyanine glucosides. Which can be upregulated by transcription factors in the preventative effect of Pueraria against cardiac fibrosis [88] or positively regulated by miRNA miR-196a-5p and miR-196b-5p [89]. These studies illustrate the regulatory complexity of DFR and UGT at the transcriptional level. In this study, we also found three lncRNAs that may regulate UGTs and DFR: MSTRG.7153.1, may positively regulate UGTs and MSTRG.3144.1 and MSTRG.3145.1 negatively regulate DFR. These regulators are another possible mechanism for the production of anthocyanins in fungi, providing a possible direction for exploring the mechanism of anthocyanins production.
Conclusions
We reported that a fungus called Aspergillus sydowii H-1 can produce anthocyanins during fermentation. This is the first report that anthocyanins can be produced in non-plant species. Through the study of the synthesis pathway, we found that CHS and C4H were typical gene existing in the strain and should contribute to fungal anthocyanin production. These findings expanded our understanding of anthocyanin biosynthesis in the nature and in deed provided a new aspect for anthocyanin metabolism.
Availability of data and materials
Supplementary files are included with this submission and contain all the information needed to reproduce the results of this study. The sequencing data generated in current study are deposited in the NCBI SRA database with the BioProject accession: PRJNA542911.
Abbreviations
- 2-ODD:
-
2-Oxoglutarate-dependent oxygenases
- 4CL:
-
4-Coumaroyl CoA ligase
- C4H:
-
Cinnamate-4-hydroxylase
- CHI:
-
Chalcone isomerase
- CHS:
-
Chalcone synthase
- CPR:
-
Cytochrome p450 reductase
- Cy:
-
Cyanidin
- DEGs:
-
Differentially expressed genes
- DFR:
-
Dihydroflavonol reductase
- DNS:
-
Dynitrosalicylic Acid
- Dp:
-
Delphinidin
- EBGs:
-
Early biosynthetic genes
- F3H:
-
Flavanone − 3- hydroxylase
- FC:
-
Fold Change
- FTIR:
-
Fourier transform infrared Spectroscopy
- G2:
-
The fermentation broth of the second day
- G8:
-
The fermentation broth of the 8th day
- KEGG:
-
Pathway Annotation of the Kyoto Encyclopedia of Genes and Genomes
- LBGs:
-
Late biosynthetic genes
- LDOX:
-
Leucoanthocyanidin dioxygenase
- lncRNA:
-
Long non-coding RNA
- Malvidin-3Ga:
-
Malvidin 3-O-galactoside
- Mv:
-
Malvidin
- NCBI:
-
National Center for Biotechnology Information
- NR:
-
Non-redundant protein sequence
- OMT:
-
O-methyltransferase
- PAL:
-
Phenylalanine lyase
- Peonidin-Mh:
-
Peonidin O-malonylhexoside
- Pg:
-
Pelargonidin
- Pn:
-
Peonidin
- Pt:
-
Petunidin
- RNAP:
-
RNA polymerase
- rRNAs:
-
Ribosomal RNAs
- TPM:
-
Transcripts Per Million
- tRNAs:
-
Transfer RNAs
- UGTs:
-
Flavonoid glycosyltransferase
- VIP:
-
Variable Importance in the Projection
References
Riaz M, Zia-Ul-Haq M, Saad B. Anthocyanins as natural colors. In: Anthocyanins and human health: biomolecular and therapeutic aspects. Cham: Springer International Publishing; 2016. p. 47–55.
Lee YM, Yoon Y, Yoon H, Park HM, Song S, Yeum KJ. Dietary Anthocyanins against Obesity and Inflammation. Nutrients. 2017:9(10).
Yousuf B, Gul K, Wani AA, Singh P. Health benefits of Anthocyanins and their encapsulation for potential use in food systems: a review. Crit Rev Food Sci Nutr. 2016;56(13):2223–30.
Xie L, Su H, Sun C, Zheng X, Wei C. Recent advances in understanding the anti-obesity activity of anthocyanins and their biosynthesis in microorganisms. Trends Food Sci Technol. 2017;72:13–24.
Holton TA, Cornish EC. Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell. 1995;7(7):1071–83.
Baek MH, Chung BY, Kim JH, Kim JS, Lee SS, An BC, Lee IJ, Kim TH. cDNA cloning and expression pattern of cinnamate-4-hydroxylase in the Korean black raspberry. BMB Rep. 2008;41(7):529–36.
Kumar R, Vashisth D, Misra A, Akhtar MQ, Jalil SU, Shanker K, Gupta MM, Rout PK, Gupta AK, Shasany AK. RNAi down-regulation of cinnamate-4-hydroxylase increases artemisinin biosynthesis in Artemisia annua. Sci Rep. 2016;6:26458.
Tengkun N, Dongdong W, Xiaohui M, Yue C, Qin C. Analysis of key genes involved in potato anthocyanin biosynthesis based on genomics and Transcriptomics data. Front Plant Sci. 2019;10:603.
Zhou H, Peng Q, Zhao J, Owiti A, Ren F, Liao L, Wang L, Deng X, Jiang Q, Han Y. Multiple R2R3-MYB transcription factors involved in the regulation of anthocyanin accumulation in peach flower. Front Plant Sci. 2016;7:1557.
Tirumalai V, Swetha C, Nair A, Pandit A, Shivaprasad PV. miR828 and miR858 regulate VvMYB114 to promote anthocyanin and flavonol accumulation in grapes. J Exp Bot. 2019.
Zheng T, Tan W, Yang H, Zhang L, Li T, Liu B, Zhang D, Lin H. Regulation of anthocyanin accumulation via MYB75/HAT1/TPL-mediated transcriptional repression. PLoS Genet. 2019;15(3):e1007993.
Wang H, Li Y, Zhang K, Ma Y, Li P. Feasibility and transcriptomic analysis of betalain production by biomembrane surface fermentation of Penicillium novae-zelandiae. AMB Express. 2018;8(1):4.
Sarang H, Rajani P, Vasanthakumari MM, Kumara PM, Siva R, Ravikanth G, Uma Shaanker R. An endophytic fungus, Gibberella moniliformis from Lawsonia inermis L. produces lawsone, an orange-red pigment. Antonie Van Leeuwenhoek. 2017;110(7):853–62.
Li D, Fu D, Zhang Y, Ma X, Gao L, Wang X, Zhou D, Zhao K. Isolation, purification, and identification of Taxol and related Taxanes from Taxol-producing fungus Aspergillus Niger subsp. taxi. J Microbiol Biotechnol. 2017;27(8):1379–85.
Mei YZ, Liu RX, Wang DP, Wang X, Dai CC. Biocatalysis and biotransformation of resveratrol in microorganisms. Biotechnol Lett. 2015;37(1):9–18.
Charles Thom MBC. The Aspergilli. In. Baltimore, Maryland: Williams & Wilkins; 1926. p. 147.
Ein-Gil N, Ilan M, Carmeli S, Smith GW, Pawlik JR, Yarden O. Presence of Aspergillus sydowii, a pathogen of gorgonian sea fans in the marine sponge Spongia obscura. Isme J. 2009;3:752.
Hallegraeff G, Coman F, Davies C, Hayashi A, McLeod D, Slotwinski A, Whittock L, Richardson AJ. Australian Dust Storm Associated with Extensive <span class="named-content genus-species" id="named-content-1">Aspergillus sydowii</span> Fungal “Bloom” in Coastal Waters. Appl Environ Microbiol. 2014;80(11):3315.
Klich MA. Identification of common Aspergillus species. Centraalbureau voor Schimmelcultures. 2002.
Geiser DM, Taylor JW, Ritchie KB, Smith GW. Cause of sea fan death in the West Indies. Nature. 1998;394(6689):137–8.
González-Abradelo D, Pérez-Llano Y, Peidro-Guzmán H, MdR S-C, Folch-Mallol JL, Aranda E, Vaidyanathan VK, Cabana H, Gunde-Cimerman N, Batista-García RA. First demonstration that ascomycetous halophilic fungi (Aspergillus sydowii and Aspergillus destruens) are useful in xenobiotic mycoremediation under high salinity conditions. Bioresour Technol. 2019;279:287–96.
Tian J, Dong Q, Yu C, Zhao R, Wang J, Chen L. Biodegradation of the organophosphate Trichlorfon and its major degradation products by a novel Aspergillus sydowii PA F-2. J Agric Food Chem. 2016;64(21):4280–7.
Alvarenga N, Birolli WG, Seleghim MHR, Porto ALM. Biodegradation of methyl parathion by whole cells of marine-derived fungi Aspergillus sydowii and Penicillium decaturense. Chemosphere. 2014;117:47–52.
Chung Y-M, Wei C-K, Chuang D-W, El-Shazly M, Hsieh C-T, Asai T, Oshima Y, Hsieh T-J, Hwang T-L, Wu Y-C, et al. An epigenetic modifier enhances the production of anti-diabetic and anti-inflammatory sesquiterpenoids from Aspergillus sydowii. Bioorg Med Chem. 2013;21(13):3866–72.
Trisuwan K, Rukachaisirikul V, Kaewpet M, Phongpaichit S, Hutadilok-Towatana N, Preedanon S, Sakayaroj J. Sesquiterpene and xanthone derivatives from the sea fan-derived fungus Aspergillus sydowii PSU-F154. J Nat Prod. 2011;74(7):1663–7.
Zhou B, Ma C, Wang H, Xia T. Biodegradation of caffeine by whole cells of tea-derived fungi Aspergillus sydowii, Aspergillus Niger and optimization for caffeine degradation. BMC Microbiol. 2018;18(1):53.
He F, Han Z, Peng J, Qian PY, Qi SH. Antifouling indole alkaloids from two marine derived fungi. Nat Prod Commun. 2013;8(3):329–32.
Zhao Ning Q, Zhang Qian, Wang Xueqing, Luo Xiuqi, Zeng Jie, Xu Hui, Cao Yi 1*: (in press) Identification of the purple pigment-producing fungus H-1 and the physicochemical property and bioactivity of pigment. Genomics Appl Biol 2018.
Miller GL. Use of Dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426–8.
Pappas CS, Takidelli C, Tsantili E, Tarantilis PA, Polissiou MG. Quantitative determination of anthocyanins in three sweet cherry varieties using diffuse reflectance infrared Fourier transform spectroscopy. J Food Compos Anal. 2011;24(1):17–21.
Huang X: Extraction, separation and chemical composition of red pigment from Adinandra millettii fruit (in Chinese). 2007.
Gu F, Wu G, Fang Y, Zhu H. Nontargeted Metabolomics for Phenolic and Polyhydroxy Compounds Profile of Pepper (Piper nigrum L.) Products Based on LC-MS/MS Analysis. Molecules. 2018;23(8).
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20.
Langmead B, Salzberg SL. Fast gapped-read alignment with bowtie 2. Nat Methods. 2012;9(4):357–9.
de Vries RP, Riley R, Wiebenga A, Aguilar-Osorio G, Amillis S, Uchima CA, Anderluh G, Asadollahi M, Askin M, Barry K, et al. Comparative genomics reveals high biological diversity and specific adaptations in the industrially and medically important fungal genus Aspergillus. Genome Biol. 2017;18(1):28.
Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357–60.
Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 2015;33(3):290–5.
Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29(7):644–52.
Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323.
Xie C, Mao X, Huang J, Ding Y, Wu J, Dong S, Kong L, Gao G, Li CY, Wei L. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011;39(Web Server issue):W316–22.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550.
Fang S, Zhang L, Guo J, Niu Y, Wu Y, Li H, Zhao L, Li X, Teng X, Sun X, et al. NONCODEV5: a comprehensive annotation database for long non-coding RNAs. Nucleic Acids Res. 2018;46(D1):D308–14.
Kang YJ, Yang DC, Kong L, Hou M, Meng YQ, Wei L, Gao G. CPC2: a fast and accurate coding potential calculator based on sequence intrinsic features. Nucleic Acids Res. 2017;45(W1):W12–6.
Sun L, Luo H, Bu D, Zhao G, Yu K, Zhang C, Liu Y, Chen R, Zhao Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013;41(17):e166.
Li A, Zhang J, Zhou Z. PLEK: a tool for predicting long non-coding RNAs and messenger RNAs based on an improved k-mer scheme. BMC Bioinformatics. 2014;15:311.
Wang G, Yin H, Li B, Yu C, Wang F, Xu X, Cao J, Bao Y, Wang L, Abbasi AA, et al. Characterization and identification of long non-coding RNAs based on feature relationship. Bioinformatics. 2019.
Tafer H, Hofacker IL. RNAplex: a fast tool for RNA-RNA interaction search. Bioinformatics. 2008;24(22):2657–63.
Li J, Ma W, Zeng P, Wang J, Geng B, Yang J, Cui Q. LncTar: a tool for predicting the RNA targets of long noncoding RNAs. Brief Bioinform. 2015;16(5):806–12.
Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, Basutkar P, Tivey ARN, Potter SC, Finn RD, et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019.
Talavera G, Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 2007;56(4):564–77.
Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–3.
Montoro P, Tuberoso CI, Perrone A, Piacente S, Cabras P, Pizza C. Characterisation by liquid chromatography-electrospray tandem mass spectrometry of anthocyanins in extracts of Myrtus communis L. berries used for the preparation of myrtle liqueur. J Chromatogr A. 2006;1112(1–2):232–40.
Goudarzi M, Mir N, Mousavi-Kamazani M, Bagheri S, Salavati-Niasari M. Biosynthesis and characterization of silver nanoparticles prepared from two novel natural precursors by facile thermal decomposition methods. Sci Rep. 2016;6:32539.
Tan C, Selig MJ, Abbaspourrad A. Anthocyanin stabilization by chitosan-chondroitin sulfate polyelectrolyte complexation integrating catechin co-pigmentation. Carbohydr Polym. 2018;181:124–31.
Fragoso S, Guasch J, Aceña L, Mestres M, Busto O. Prediction of red wine colour and phenolic parameters from the analysis of its grape extract. Int J Food Sci Technol. 2011;46(12):2569–75.
Sulekova M, Hudak A, Smrcova M. The Determination of Food Dyes in Vitamins by RP-HPLC. Molecules. 2016;21(10).
Tatsuzawa F, Tanikawa N, Nakayama M. Red-purple flower color and delphinidin-type pigments in the flowers of Pueraria lobata (Leguminosae). Phytochemistry. 2017;137:52–6.
Basalekou M, Pappas C, Kotseridis Y, Tarantilis PA, Kontaxakis E, Kallithraka S. Red wine age estimation by the alteration of its color parameters: Fourier transform infrared spectroscopy as a tool to monitor wine maturation time. J Anal Methods Chem. 2017;2017:5767613.
Tsutsumi A, Horikoshi Y, Fushimi T, Saito A, Koizumi R, Fujii Y, Hu QQ, Hirota Y, Aizawa K, Osakabe N. Acylated anthocyanins derived from purple carrot (Daucus carota L.) induce elevation of blood flow in rat cremaster arteriole. Food Funct. 2019;10(3):1726–35.
Yang L, Rong-Rong C, Ji-Li F, Ke Y. Total anthocyanins and cyanidin-3-O-glucoside contents and antioxidant activities of purified extracts from eight different pigmented plants. Pharmacogn Mag. 2019;15(60):124.
Dong T, Han R, Yu J, Zhu M, Zhang Y, Gong Y, Li Z. Anthocyanins accumulation and molecular analysis of correlated genes by metabolome and transcriptome in green and purple asparaguses (Asparagus officinalis, L.). Food Chem. 2019;271:18–28.
Polturak G, Heinig U, Grossman N, Battat M, Leshkowitz D, Malitsky S, Rogachev I, Aharoni A. Transcriptome and metabolic profiling provides insights into Betalain biosynthesis and evolution in Mirabilis Jalapa. Mol Plant. 2018;11(1):189–204.
Pelletier MK, Murrell JR, Shirley BW. Characterization of flavonol synthase and leucoanthocyanidin dioxygenase genes in Arabidopsis. Further evidence for differential regulation of “early” and “late” genes. Plant Physiol. 1997;113(4):1437–45.
Ferrer JL, Austin MB, Stewart C Jr, Noel JP. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol Biochem. 2008;46(3):356–70.
Ma L, Bajic VB, Zhang Z. On the classification of long non-coding RNAs. RNA Biol. 2013;10(6):925–33.
Wang HV, Chekanova JA. Long noncoding RNAs in plants. Adv Exp Med Biol. 2017;1008:133–54.
Wang J, Meng X, Dobrovolskaya OB, Orlov YL, Chen M. Non-coding RNAs and their roles in stress response in plants. Genomics Proteomics Bioinformatics. 2017;15(5):301–12.
Weitz JI, Hirsh J, Samama MM. New anticoagulant drugs: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126(3 Suppl):265S–86S.
Overall J, Bonney SA, Wilson M, Beermann A, Grace MH, Esposito D, Lila MA, Komarnytsky S. Metabolic Effects of Berries with Structurally Diverse Anthocyanins. Int J Mol Sci. 2017;18(2).
Chen XQ, Nagao N, Itani T, Irifune K. Anti-oxidative analysis, and identification and quantification of anthocyanin pigments in different coloured rice. Food Chem. 2012;135(4):2783–8.
Silva GG, Dutra M, de Oliveira JB, Rybka ACP, Pereira GE, Dos Santos LM. Processing methods with heat increases bioactive phenolic compounds and antioxidant activity in grape juices. J Food Biochem. 2019;43(3):e12732.
Wei H, Li H, Wan SP, Zeng QT, Cheng LX, Jiang LL, Peng YD. Cardioprotective effects of Malvidin against isoproterenol-induced myocardial infarction in rats: a mechanistic study. Med Sci Monit. 2017;23:2007–16.
Seo HR, Choi MJ, Choi JM, Ko JC, Ko JY, Cho EJ. Malvidin protects WI-38 human fibroblast cells against stress-induced premature senescence. J Cancer Prev. 2016;21(1):32–40.
Dai T, Shi K, Chen G, Shen Y, Pan T. Malvidin attenuates pain and inflammation in rats with osteoarthritis by suppressing NF-kappaB signaling pathway. Inflamm Res. 2017;66(12):1075–84.
Wang C, Fu D. Virus-induced gene silencing of the eggplant Chalcone synthase gene during fruit ripening modifies epidermal cells and Gravitropism. J Agric Food Chem. 2018;66(11):2623–9.
Birney E, Clamp M, Durbin R. GeneWise and Genomewise. Genome Res. 2004;14(5):988–95.
Fitzpatrick DA. Horizontal gene transfer in fungi. FEMS Microbiol Lett. 2012;329(1):1–8.
Emiliani G, Fondi M, Fani R, Gribaldo S. A horizontal gene transfer at the origin of phenylpropanoid metabolism: a key adaptation of plants to land. Biol Direct. 2009;4:7.
Richards TA, Soanes DM, Foster PG, Leonard G, Thornton CR, Talbot NJ. Phylogenomic analysis demonstrates a pattern of rare and ancient horizontal gene transfer between plants and fungi. Plant Cell. 2009;21(7):1897–911.
Li M, Zhao J, Tang N, Sun H, Huang J. Horizontal gene transfer from Bacteria and plants to the Arbuscular Mycorrhizal fungus Rhizophagus irregularis. Front Plant Sci. 2018;9:701.
Martin FM, Uroz S, Barker DG. Ancestral alliances: Plant mutualistic symbioses with fungi and bacteria. Science. 2017;356(6340).
Moller M, Stukenbrock EH. Evolution and genome architecture in fungal plant pathogens. Nat Rev Microbiol. 2017;15(12):756–71.
Wang Z, Yan S, Liu C, Chen F, Wang T. Proteomic analysis reveals an aflatoxin-triggered immune response in cotyledons of Arachis hypogaea infected with Aspergillus flavus. J Proteome Res. 2012;11(5):2739–53.
Gupta VK: New and future developments in microbial biotechnology and bioengineering. Aspergillus system properties and applications. Amsterdam, Boston: Elsevier; 2016.
Levy A, Salas Gonzalez I, Mittelviefhaus M, Clingenpeel S, Herrera Paredes S, Miao J, Wang K, Devescovi G, Stillman K, Monteiro F, et al. Genomic features of bacterial adaptation to plants. Nat Genet. 2017;50(1):138–50.
Landi M, Tattini M, Gould KS. Multiple functional roles of anthocyanins in plant-environment interactions. Environ Exp Bot. 2015;119:4–17.
Plunkett BJ, Espley RV, Dare AP, Warren BAW, Grierson ERP, Cordiner S, Turner JL, Allan AC, Albert NW, Davies KM, et al. MYBA from blueberry (Vaccinium section Cyanococcus) is a subgroup 6 type R2R3MYB transcription factor that activates anthocyanin production. Front Plant Sci. 2018;9:1300.
Cai SA, Hou N, Zhao GJ, Liu XW, He YY, Liu HL, Hua YQ, Li LR, Huang Y, Ou CW, et al. Nrf2 is a key regulator on Puerarin preventing cardiac fibrosis and Upregulating metabolic enzymes UGT1A1 in rats. Front Pharmacol. 2018;9:540.
Sutliff AK, Watson CJW, Chen G, Lazarus P. Regulation of UGT2A1 by miR-196a-5p and miR-196b-5p. J Pharmacol Exp Ther. 2019;369(2):234–43.
Acknowledgments
Not Applicable.
Funding
This work was supported by National Natural Science Foundation of China (31670078); Sichuan Science and Technology Program (2018NZ0007, 2019YJ0023 and 2019JDPT0012); Chengdu Science and Technology Program (2017-GH02–00071-HZ and 2018-YF05–00738-SN); National Infrastructure of Natural Resources for Science and Technology Program of China (NIMR-2018-8); Fundamental Research Funds for the Central Universities (2018SCUH0072). The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
CFB and JZ analyzed the data and drafted the manuscript. QZ performed laboratory experiments. CFB and ZNH participated in the data analysis and revised manuscript. DRQ and XYC participated in the sample collection. YC and HX conceived the study and revised the manuscript. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
Raw data of the time course of biomass, sugar consumption and crude pigment content
Additional file 2: Table S2.
Metabolome data used in this study. Lists of compounds include ion mode, Rt (min), molecular weight (Da), compounds, class, MEAN value at G2 and G8, VIP, P-value, and fold change
Additional file 3: Table S3.
Differentially expressed genes (DEGs) (P-value < 0.5 and log2FC > 1) in G2 and G8. Lists of DEGs include gene ID, length, log2FC, P-value, count, and annotation (XLS 694 kb)
Additional file 4: Table S4.
Primers used in this study
Additional file 5: Table S5.
CHS and C4H blast results show that the plant-derived gene may be responsible for the production of anthocyanins. Lists of the blast results include gene symbols, query names, subject names, subject species, identity, alignment length, mismatch length, gap openings, query start, query end, subject start, subject end, e-value and score
Additional file 6: Figure S1.
OPLS-DA score plots generated from OPLS-DA models and different metabolites (DMs) between two time-points. The parameters for the classification were R2Y = 0.98 and Q2Y = 0.99, which were stable and good to fitness and prediction. Figure S2. Permutation test was proceeded in order to further validate the model. The R and Q intercept values were 0.35 and − 1.25 after 200 permutations. The low values of Q intercept indicate the robustness of the models, and thus show a low risk of over fitting and reliable. Figure S3. The PCA results show that the quality control samples have good repeatability, and the sample mass spectrometry monitoring analysis is stable, and the data repeatability and credibility are high. Figure S4. Heatmap of the correlations among samples.
Additional file 7.
Total transcript fasta sequences obtained in this study
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bu, C., Zhang, Q., Zeng, J. et al. Identification of a novel anthocyanin synthesis pathway in the fungus Aspergillus sydowii H-1. BMC Genomics 21, 29 (2020). https://doi.org/10.1186/s12864-019-6442-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-019-6442-2