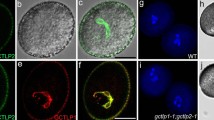
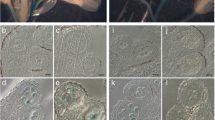
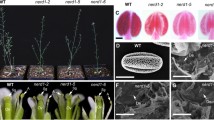
Abstract
Pollination is essential for seed reproduction and for exchanges of genetic information between individual plants. In angiosperms, mature pollen grains released from dehisced anthers are transferred to the stigma where they become hydrated and begin to germinate. Pollen grains of wild-type Arabidopsis thaliana do not germinate inside the anther under normal growth conditions. We report two Arabidopsis lines that produced pollen grains able to in situ precociously germinate inside the anther. One of them was a callose synthase 9 (cs9) knockout mutant with a T-DNA insertion in the Callose Synthase 9 gene (CalS9). Male gametophytes carrying a cs9 mutant allele were defective and no homozygous progeny could be produced. Heterozygous mutant plants (cs9/+) produced approximately 50% defective pollen grains with an altered male germ unit (MGU) and aberrant callose deposition in bicellular pollen. Bicellular pollen grains germinated precociously inside the anther. Another line, a transgenic plant expressing callose synthase 5 (CalS5) under the CaMV 35S promoter, also contained abnormal callose deposition during microsporogenesis and displaced MGUs in pollen grains. We also observed that precocious pollen germination could be induced in wild-type plants by incubation with medium containing sucrose and calcium ion and by wounding in the anther. These results demonstrate that precocious pollen germination in Arabidopsis could be triggered by a genetic alteration and a physiological condition.








Similar content being viewed by others
Abbreviations
- CalS:
-
Callose synthase
- GSL:
-
Glucan synthase-like
- MGU:
-
Male germ unit
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- DAPI:
-
4′6-Diamidino-2-phenylindole
References
Alexander MP (1969) Differential staining of aborted and nonaborted pollen. Stain Technol 44:117–122
Chen XY, Liu L, Lee E, Han X, Rim Y, Chu H, Kim SW, Sack F, Kim JY (2009) The Arabidopsis callose synthase gene GSL8 is required for cytokinesis and cell patterning. Plant Physiol 150:105–113
Culley TM, Klooster MR (2007) The cleistogamous breeding system: a review of its frequency, evolution, and ecology in angiosperms. Bot Rev 73:1–30
Dong J, Kim ST, Lord EM (2005a) Plantacyanins plays a role in reproduction in Arabidopsis. Plant Physiol 138:778–789
Dong X, Hong Z, Sivaramakrishnan M, Mahfouz M, Verma DPS (2005b) Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. Plant J 42:315–328
Dong X, Hong Z, Chatterjee J, Kim S, Verma DPS (2008) Expression of callose synthase genes and its connection with Npr1 signaling pathway during pathogen infection. Planta 229:87–98
Edlund AF, Swanson R, Preuss D (2004) Pollen and stigma structure and function: the role of diversity in pollination. Plant Cell 16:S84–S97
Enns LC, Kanaoka MM, Torii KU, Comai L, Okada K, Cleland RE (2005) Two callose synthases, GSL1 and GSL5, play an essential and redundant role in plant and pollen development and in fertility. Plant Mol Biol 58:333–349
Eyüboglu B, Pfister K, Haberer G, Chevalier D, Fuchs A, Mayer KF, Schneitz K (2007) Molecular characterization of the STRUBBELIG-RECEPTOR FAMILY of genes encoding putative leucine-rich repeat receptor-like kinases in Arabidopsis thaliana. BMC Plant Biol 7:16
Fan LM, Wang YF, Wang H, Wu WH (2001) In vitro Arabidopsis pollen germination and characterization of the inward potassium currents in Arabidopsis pollen grain protoplasts. J Exp Bot 52:1603–1614
Hiscock SJ, Allen AM (2008) Diverse cell signalling pathways regulate pollen–stigma interactions: the search for consensus. New Phytol 179:286–317
Hong Z, Delauney AJ, Verma DPS (2001) A cell-plate specific callose synthase and its interaction with phragmoplastin. Plant Cell 13:755–768
Huang L, Chen XY, Rim Y, Han X, Cho WY, Kim SW, Kim JY (2009) Arabidopsis glucan synthase-like 10 functions in male gametogenesis. J Plant Physiol 166:344–352
Jacobs AK, Lipka V, Burton RA, Panstruga R, Strizhov N, Schulze-Lefert P, Fincher GBJ (2003) An Arabidopsis callose synthase, GSL5, is required for wound and papillary callose formation. Plant Cell 15:2503–2513
Jenik PD, Irish VF (2000) Regulation of cell proliferation patterns by homeotic genes during Arabidopsis floral development. Development 127:1267–1276
Johnson SA, McCormick S (2001) Pollen germinates precociously in the anthers of raring-to-go, an Arabidopsis gametophytic mutant. Plant Physiol 126:685–695
Johnson-Brousseau SA, McCormick S (2004) A compendium of methods useful for characterizing Arabidopsis pollen mutants and gametophytically expressed genes. Plant J 39:761–775
Kandasamy MK, Nasrallah JB, Nasrallah ME (1994) Pollen–pistil interactions and developmental regulation of pollen tube growth in Arabidopsis. Development 12:3405–3418
Ko JH, Kim JH, Jayanty SS, Howe GA, Han KH (2006) Loss of function of COBRA, a determinant of oriented cell expansion, invokes cellular defense responses in Arabidopsis thaliana. J Exp Bot 57:2923–2936
Lolle SJ, Cheung AY (1993) Promiscuous germination and growth of wild type pollen from Arabidopsis and related species on the shoot of the Arabidopsis mutant, fiddlehead. Dev Biol 155:250–258
Lord EM (1979) The development of cleistogamous and chasmogamous flowers in Lamium amplexicaule (Labiatae): an example of heteroblastic inflorescence development. Bot Gaz 140:39–50
Lord EM (1981) Cleistogamy: a tool for the study of floral morphogenesis, function and evolution. Bot Rev 47:421–449
McCormick S (2004) Control of male gametophyte development. Plant Cell 16:S142–S153
Nishikawa S, Zinkl GM, Swanson RJ, Maruyama D, Preuss D (2005) Callose (beta-1,3 glucan) is essential for Arabidopsis pollen wall patterning, but not tube growth. BMC Plant Biol 5:22
Nishimura MT, Stein M, Hou BH, Vogel JP, Edwards H, Somerville SC (2003) Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 301:969–972
Park SK, Twell D (2001) Novel patterns of ectopic cell plate growth and lipid body distribution in the Arabidopsis Gemini pollen1 mutant. Plant Physiol 126:899–909
Paxson-Sowders DM, Owen HA, Makaroff CA (1997) A comparative ultrastructural analysis of exine pattern development in wild-type Arabidopsis and a mutant defective in pattern formation. Protoplasma 198:53–65
Preuss D, Rhee SY, Davis RW (1994) Tetrad analysis possible in Arabidopsis with mutation of the Quartet (Qrt) genes. Science 264:1458–1460
Sanchez AM, Bosch M, Bots M, Nieuwland J, Feron R, Mariani C (2004) Pistil factors controlling pollination. Plant Cell 16:S98–S106
Shedletzky E, Unger C, Delmer DP (1997) A microtiter-based fluorescence assay for (1,3)-beta-glucan synthases. Anal Biochem 249:88–93
Singh DP, Jermakow AM, Swain SM (2002) Gibberellins are required for seed development and pollen tube growth in Arabidopsis. Plant Cell 14:3133–3147
Stone BA, Clarke AE (1992) Chemistry and physiology of higher plant 1,3-β-glucans (callose). In: Stone BA, Clarke AE (eds) Chemistry and biology of (1, 3)-β-glucans. La Trobe University Press, Melbourne, pp 365–429
Taylor LP, Hepler PK (1997) Pollen germination and tube growth. Annu Rev Plant Physiol Plant Mol Biol 48:461–491
Thiele K, Wanner G, Kindzierski V, Jürgens G, Mayer U, Pachl F, Assaad FF (2008) The timely deposition of callose is essential for cytokinesis in Arabidopsis. Plant J 58:13–26
Töller A, Brownfield L, Neu C, Twell D, Schulze-Lefert P (2008) Dual function of Arabidopsis glucan synthase-like genes GSL8 and GSL10 in male gametophyte development and plant growth. Plant J 54:911–923
Van Damme D, Coutuer S, De Rycke R, Bouget FY, Inze D, Geelen D (2006) Somatic cytokinesis and pollen maturation in Arabidopsis depend on TPLATE, which has domains similar to coat proteins. Plant Cell 18:3502–3518
Verma DPS, Hong Z (2001) Plant callose synthase complexes. Plant Mol Biol 47:693–701
Vithanage HI, Gleeson PA, Clarke AE (1980) The nature of callose produced during self-pollination in Secale cereale. Planta 148:498–509
Wilkinson JE, Twell D, Lindsey K (1997) Activities of CaMV 35S and NOS promoters in pollen: implications for field release of transgenic plants. J Exp Bot 48:265–275
Witte CP, Noel LD, Gielbert J, Parker JE, Romeis T (2004) Rapid one-step protein purification from plant material using the eight-amino acid StrepII epitope. Plant Mol Biol 55:135–147
Worrall D, Hird DL, Hodge R, Paul W, Draper J, Scott R (1992) Premature dissolution of the microsporocyte callose wall causes male sterility in transgenic tobacco. Plant Cell 4:759–771
Zhang ZB, Zhu J, Gao JF, Wang C, Li H, Zhang HQ, Zhang S, Wang DM, Wang QX, Huang H, Xia HJ, Yang ZN (2007) Transcription factor AtMYB103 is required for anther development by regulating tapetum development, callose dissolution and exine formation in Arabidopsis. Plant J 52:528–538
Zhu J, Chen H, Li H, Gao JF, Jiang H, Wang C, Guan YF, Yang ZN (2008) Defective in Tapetal Development and Function 1 is essential for anther development and tapetal function for microspore maturation in Arabidopsis. Plant J 55:266–277
Acknowledgments
We thank the Arabidopsis Biological Resources Center (ABRC) for providing Arabidopsis seeds. This work was supported by grants from NSF (MCB 0548525 and IOB 0543923).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
425_2009_1091_MOESM1_ESM.tif
Supplemental Fig. S1 Genomic PCR of cs9-5 and cs9-6 T-DNA insertion mutants. a Map of T-DNA insertion sites and primers used for genomic PCR reactions. There are two T-DNA inserts in cs9-5 (Salk_060111) and one in cs9-6 (Salk_124294). The directions of primers and T-DNA inserts are indicated by arrows. Black boxes represent exons and open boxes represent T-DNA. Primers used for genomic PCR reactions are LP 5′-ATA TAC CCT TGC ACC ACC ATG-3′, RP 5′-AAT ACT TAG CAG TTA GCA GGC G-3′ and LBb1 (Lb) 5′-GCG TGG ACC GCT TGC TGC AAC T-3′. b Genomic PCR products using two primers as indicated. In wild type, the amplified PCR products were a 1.05 kb genomic fragment from wild-type plants, two T-DNA specific fragments (0.8 and 0.5 kb) for cs9-5 and one T-DNA specific product (0.2 kb) for cs9-6. A nonspecific amplification product of 0.45 kb, indicated by star (*), was present in reactions with the LBb1 primer(TIFF 220 kb)
425_2009_1091_MOESM2_ESM.tif
Supplemental Fig. S2 Defects in reproduction in Arabidopsis plants expressing CalS5. a Phenotypes of newly opened anthers of wild type (WT), transgenic plants (S-CS5/WT, line 3) and cs5-1 mutant (cs5-1). Note that anthers of the transgenic plants and cs5-1 mutant were shrunken and contained fewer pollen grains. Bar, 0.5 mm b Silique phenotypes of wild type, S-CS5/WT transgenic plants (line 3) and cs5-1 mutant. Bar, 10 mm. c Embryos in the siliques of wild type, S-CS5/WT transgenic plants and cs5-1 mutant. Bar, 2.5 mm. d Differential interference contrast (DIC) microscope images of pollen grains of wild type (WT), S-CS5/WT plants (line 3) and cs5-1 mutant. Bar, 5 μm. e Scanning electron microscopy (SEM) images of pollen grains of wild type, and 3S-CS5 /WT plants (line 3). Bar, 5 μm (TIFF 3100 kb)
425_2009_1091_MOESM3_ESM.tif
Supplemental Fig. S3 Expression of CalS genes, callose contents and aborted embryos in transgenic plants expressing S-CS5. a-b RT-PCR analysis of the expression of CalS5 gene and the transgene S-CS5 in the leaf (a) and root (b) of 5 randomly selected S-CS5/WT transgenic plants. Primer CS5F2 and CS5R2 were used to detect the mRNA of CalS5. Primer CS5F1 corresponding to the CalS5 N-terminal region and the Strep specific primer StpR were used to detect the mRNA of S-CS5, Actin2 mRNA was used as an internal control. Three replications were conducted and one representative result is shown. c Callose contents of the tissues of wild type, cs5-1 mutant and S-CS5/WT (left-right: line 3, 5, 9, 11 and 14) plants. Quantitative measurements of callose content were performed as described previously (Shedletzky et al. 1997). Error bars were the means ± standard deviations from three replicate assays. In each plant tissues, Columns marked with different letters were significantly different according to Duncan multirange test, p≤ 0.05. d-e Percentages of short siliques (less than 5 mm), and aborted embryos in wild type, cs5-1 mutant and S-CS5/WT (left-right: line 3, 5, 9, 11 and 14) plants. Error bars were the means ± standard deviations from three replicate assays. Columns marked with different letters were significantly different according to Duncan multirange test, p≤ 0.05(TIFF 716 kb)
Rights and permissions
About this article
Cite this article
Xie, B., Wang, X. & Hong, Z. Precocious pollen germination in Arabidopsis plants with altered callose deposition during microsporogenesis. Planta 231, 809–823 (2010). https://doi.org/10.1007/s00425-009-1091-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-009-1091-3