Abstract
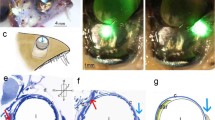
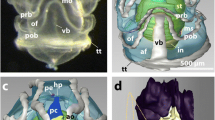
The compound eyes of ark clams appear to function as an optical system to trigger shell closure against predators. We have analyzed the structure of the ommatidia of Arca noae by thin section electron microscopy and serial sectioning, Concanavalin A–gold labeling and acid phosphatase cytochemistry. Our results demonstrate that the ommatidia are a three-tier structure composed of a central single receptor cell, surrounded and covered by proximal pigment cells followed by rows of distal pigment cells. The receptor cells of Arca noae have no lens and the disks of their receptive segment are derived from sensory cilia. The distal mitochondrial segment in the cytoplasm between the nucleus and the receptive segment is surrounded by a mass of Concanavalin A-reactive glycogen particles. Although both, proximal and distal pigment cells have numerous microvilli, only those of the proximal pigment cells form a well-aligned brush border. The microvilli of the latter are ≈9–11 μm long and have a diameter of ≈70–80 nm. Numerous microlamellar bodies cover them. The microlamellar bodies are stored in acid phosphatase-negative secretory granules of the pigment granule-free apical cytoplasm of proximal pigment cells before their secretion. Observation of living compound eyes indicated that the apex of proximal pigment cells transmitted significantly more light than the surrounding distal pigment cells. Hence, the regular geometry of the brush border seems to be a light-guiding structure for receptor cells similar to an optical fiber.








Similar content being viewed by others
References
Dietl P, Haller T (2005) Exocytosis of lung surfactant: from the secretory vesicle to the air–liquid interface. Annu Rev Physiol 67:595–621
Eakin R, Brandenburger J (1975) Understanding a snail’s eye at a snails’s pace. Am Zool 15:851–863
Elias PM, Menon GK (1991) Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv Lipid Res 24:1–26
Franze K, Grosche J, Skatchkov SN, Schinkinger S, Foja C, Schild D, Uckermann O, Travis K, Reichenbach A, Guck J (2007) Muller cells are living optical fibers in the vertebrate retina. Proc Natl Acad Sci USA 104:8287–8292
Gehring WJ (2005) New perspectives on eye development and the evolution of eyes and photoreceptors. J Hered 96:171–184
Gehring WJ, Ikeo K (1999) Pax 6: mastering eye morphogenesis and eye evolution. Trends Genet 15:371–377
Goldstein IJ, Poretz RD (1986) Isolation, physicochemical characterization, and carbohydrate-binding specificity of lectins. In: Liener IE, Sharon N, Goldstein IJ (eds) The lectins. Properties, functions and applications in biology and medicine. Academic Press, Orlando, pp 35–247
Graziussi D, Suga H, Schmid V, Gehring W (2011) Eyes absent in the eye-bearing hydrozoan jellyfish Cladonema radiatum: conservation of the retinal determination network. J Exp Zool (in press)
Hayat M (2000) Principles and techniques of electron microscopy. Biological applications. Cambridge University Press, Cambridge
Karnovsky M (1971) Use of ferrocyanide-reduced osmium tetroxide in electron microscopy. Abstracts of fourteenth annual meeting. Am Soc Cell Biol 114
Katagiri N (1984) Cytoplasmic characteristics of three different rhabdomeric photoreceptor cells in a marine gastropod, Onchidium verruculatum. J Electron Microsc (Tokyo) 33:142–150
Kerneis A (1973) Etude comparée d’organes photorécepteurs de Sabellidae (Annélides Polychètes). J Ultrastruct Res 53:164–179
Kozmik Z, Daube M, Frei E, Norman B, Kos L, Dishaw LJ, Noll M, Piatigorsky J (2003) Role of Pax genes in eye evolution: a cnidarian PaxB gene uniting Pax2 and Pax6 functions. Dev Cell 5:773–785
Lee M, Jago J, Garcia-Bellido D, Edgecombe G, Gehling J, Paterson J (2011) Modern optics in exceptionally preserved early Cambrian arthropod. Nature (in press)
Madison KC (2003) Barrier function of the skin: ‘‘La Raison d’Etre’’ of the epidermis. J Invest Dermatol 121:231–241
Maunsbach A, Afzelius B (1999) Biomedical electron microscopy. Illustrated methods and interpretations. Academic Press, San Diego
Nilsson DE (1994) Eyes as optical alarm systems in fan worms and ark clams. Philos Trans R Soc Lond B 346:195–212
Oashi M, Sawada Y, Makita R (1973) Odland body and intercellular substances. Acta Derm Venereol Suppl (Stockholm) 73:47–54
Orgeig s, Daniels CB, Sullivan LC (2004) Development of the pulmonary surfactant system. In: Harding R, Pinkerton K, Plopper CG (eds) The lung: development aging and the environment. Elsevier, Amsterdam, pp 149–167
Pavelka M, Roth J (2010) Functional ultrastructure. An atlas of tissue biology and pathology, 2nd edn. Springer, Vienna
Robinson J, Karnovsky M (1983) Ultrastructural localization of several phosphatases with cerium. J Histochem Cytochem 31:1197–1208
Roth J (1983) Application of lectin–gold complexes for electron microscopic localization of glycoconjugates on thin sections. J Histochem Cytochem 31:987–999
Roth J, Bendayan M, Orci L (1978) Ultrastructural localization of intracellular antigens by the use of protein A–gold complex. J Histochem Cytochem 26:1074–1081
Suga H, Tschopp P, Graziussi DF, Stierwald M, Schmid V, Gehring WJ (2010) Flexibly deployed Pax genes in eye development at the early evolution of animals demonstrated by studies on a hydrozoan jellyfish. Proc Natl Acad Sci USA 107:14263–14268
Tokuyasu K (1978) A study of positive staining of ultrathin frozen sections. J Ultrastruct Res 63:287–307
Tokuyasu K (1986) Application of cryoultramicrotomy to immunocytochemistry. J Microsc (Oxf) 143:139–149
Vanhecke D, Herrmann G, Graber W, Hillmann-Marti T, Muhlfeld C, Studer D, Ochs M (2010) Lamellar body ultrastructure revisited: high-pressure freezing and cryo-electron microscopy of vitreous sections. Histochem Cell Biol 134:319–326
Williams TJ, Homer LD, Shafer JA, Goldstein IJ, Garegg PJ, Hultberg H, Iversen T, Johansson R (1981) Characterization of the extended carbohydrate binding site of Concanavalin A: specificity for interaction with the nonreducing termini of alpha-(1, 2)-linked disaccharides. Arch Biochem Biophys 209:555–564
Acknowledgments
We thank Mrs. Annette Roulier for excellent artwork. This work was supported by World Class University (WCU) program through the National Research Foundation (NRF) of Korea funded by the Ministry of Education, Science and Technology (MEST) (Grant no. R31-10086), the Kantons of Zurich, Basel and Basel-Landschaft, Switzerland and the Swiss National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
418_2011_828_MOESM1_ESM.tif
Supplemental Fig. 1. (A-C) Microvilli of proximal pigment cells form an aligned brush border (BB), whereas those of the distal pigment cells (DPC) are loosely arranged (MV). RC: receptor cells. Scale bars: 2.5 μm (A), 0.9 μm (B), 0.36 μm (C). (TIFF 5107 kb)
418_2011_828_MOESM2_ESM.tif
Supplemental Fig. 2. (A) Details from the apical cytoplasm of receptor cells containing a Golgi apparatus (GA) with associated small vesicles (arrowheads) as well as glycogen particles (asterisks). (B) Some mitochondria of the distal mitochondrial segment (DMS). The space between them and the lateral plasma membrane is filled with glycogen particles (asterisk). Arrowheads point to small vesicles in the rim of cytoplasm. Scale bars: 0.27 μm (A), 0.14 μm (B) (TIFF 3421 kb)
418_2011_828_MOESM3_ESM.tif
Supplemental Fig. 3. High resolution micrographs of longitudinal (A) and cross (B) sections from the brush border of proximal pigment cells with its microlamellar bodies. Scale bars: 0.1 μm (A), 0.19 μm (B) (TIFF 3669 kb)
418_2011_828_MOESM4_ESM.tif
Supplemental Fig. 4. Longitudinal (A) and oblique (B) sections of the brush border of proximal pigment cells. The uppermost part and the tips of the microvilli are free of microlamellar bodies, which becomes very obvious in the oblique sectioned brush border. Scale bars: 88 nm (A), 100 nm (B) (TIFF 5152 kb)
418_2011_828_MOESM5_ESM.tif
Supplemental Fig. 5. (A-C) Details from proximal pigment cells (PPC) with clusters of large vesicles (V), which contain microlamellar bodies. This part of the cytoplasm of the PPCs indents in a receptor cell (RC). BB: brush border of PPC. Gly: glycogen particles of RC. Scale bars: 0.35 μm (A, B), 0.28 μm (C) (TIFF 4754 kb)
Rights and permissions
About this article
Cite this article
Roth, J., Guhl, B., Kloter, U. et al. The ommatidia of Arca noae: a three-tier structure with a central light-guiding element for the receptor cell. Histochem Cell Biol 136, 11–23 (2011). https://doi.org/10.1007/s00418-011-0828-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-011-0828-9