Abstract
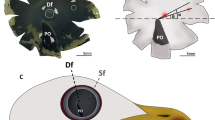
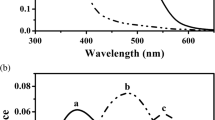
The retinal structure of the booted eagle (Aquila pennata) was investigated using light and electron microscopy. Particular attention is paid to the main ultrastructural features of the receptor cells. This study reveals six distinct varieties of cones. Unequal double cones differ in shape, structure, and length and are comprised by principal long and accessory short members. Principal member contains a green oil droplet and accessory member contains a paraboloid and a pale green droplet. Four types of single cones are distinguished on the basis of their morphology and oil droplets: red, green, blue, and ultraviolet. Cones outnumber rods in all regions. Two types of horizontal cells and several morphological types of amacrine cells are abundant. A large number of bipolar cells are divided into long longitudinal rows by Müller cell processes, a prominent feature of this retina. These processes extend through the external limiting membrane to reach the ellipsoid region of the cones. Moreover, thick processes divide the inner nuclear and plexiform layers and surround the myelinated ganglion cell axons at fairly regular intervals. In the ganglion cell layer and optic nerve fibre layer, abundant oligodendrocytes are present, close to the myelinated axons. The morphological characteristics of this retina indicate that A. pennata have good colour discrimination, a complex visual processing to mediate contrast and motion and an elevated acuity in areas of high cell densities.






Similar content being viewed by others
References
Beason RC, Loew ER (2008) Visual pigment and oil droplet characteristics of the bobolink (Dolichonyx oryzivorus), a new world migratory bird. Vis Res 48(1):1–8
Bowmaker JK (1977) The visual pigments, oil droplets and spectral sensitivity of the pigeon. Vis Res 17(10):1129–1138
Bowmaker JK, Martin GR (1978) Visual pigments and colour vision in a nocturnal bird, Strix aluco (tawny owl). Vis Res 18(9):1125–1130
Bowmaker JK, Heath LA, Wilkie SE, Hunt DM (1997) Visual pigments and oil droplets from six classes of photoreceptor in the retinas of birds. Vis Res 37(16):2183–2194
Braekevelt CR (1992) Retinal pigment epithelial fine structure in the red-tailed hawk (Buteo jamaicensis). Anat Histol Embryol 21(1):48–56
Braekevelt CR (1993a) Retinal photoreceptor fine structure in the red-tailed hawk (Buteo jamaicensis). Anat Histol Embryol 22(3):222–232
Braekevelt CR (1993b) Fine structure of the retinal photoreceptors of the great horned owl (Bubo virginianus). Histol Histopathol 8(1):25–34
Braekevelt CR, Thorlakson IJ (1993) Fine structure of the retinal pigment epithelium of the great horned owl (Bubo virginianus). Histol Histopathol 8(1):17–23
Braekevelt CR, Smith BJ, Smith SA (1996a) Fine structure of the retinal pigment epithelium of the barred owl (Strix varia). Histol Histopathol 11(1):71–77
Braekevelt CR, Smith SA, Smith BJ (1996b) Fine structure of the retinal photoreceptors of the barred owl (Strix varia). Histol Histopathol 11(1):79–88
Bravo H, Pettigrew JD (1981) The distribution of neurons projecting from the retina and visual cortex to the thalamus and tectum opticum of the barn owl, Tyto alba, and the burrowing owl, Speotyto cunicularia. J Comp Neurol 199(3):419–441
Budnik V, Mpodozis J, Varela FJ, Maturana HR (1984) Regional specialization of the quail retina: ganglion cell density and oil droplet distribution. Neurosci Lett 51(1):145–150
Campenhausen MV, Kirschfeld K (1998) Spectral sensitivity of the accessory optic system of the pigeon. J Comp Physiol A 183(1):1–6
Cowan WM, Powell TP (1963) Centrifugal fibres in the avian visual system. Proc R Soc Lond B Biol Sci 158:232–252
Cuthill IC, Partridge JC, Bennett ATD, Church SC, Hart NS, Hunt S (2000) Ultraviolet vision in birds. Adv Stud Behav 29:159–214
Dolan T, Fernández-Juricic E (2010) Retinal ganglion cell topography of five species of ground-foraging birds. Brain Behav Evol 75(2):111–121
Dontsov AE, Glickman RD, Ostrovsky MA (1999) Retinal pigment epithelium pigment granules stimulate the photo-oxidation of unsaturated fatty acids. Free Radic Biol Med 26(11–12):1436–1446
Edmonds DT (1996) A sensitive optically detected magnetic compass for animals. Proc Biol Sci 263(1368):295–298
Ehrlich D (1981) Regional specialization of the chick retina as revealed by the size and density of neurons in the ganglion cell layer. J Comp Neurol 195(4):643–657
El-Beltagy AEFB (2015) Light and electron microscopic studies on the pigmented epithelium and photoreceptors of the retina of common buzzard (Buteo buteo). Tissue Cell 47(1):78–85
Fischer AJ, Stell WK (1999) Nitric oxide synthase-containing cells in the retina, pigmented epithelium, choroid, and sclera of the chick eye. J Comp Neurol 405(1):1–14
Fritzsch B, Crapon de Caprona MD, Clarke PG (1990) Development of two morphological types of retinopetal fibers in chick embryos, as shown by the diffusion along axons of a carbocyanine dye in the fixed retina. J Comp Neurol 300(3):405–421
Gaffney MF, Hodos W (2003) The visual acuity and refractive state of the American kestrel (Falco sparverius). Vis Res 43(19):2053–2059
Gallego A, Baron M, Gayoso M (1975) Organization of the outer plexiform layer of the diurnal and nocturnal bird retinae. Vis Res 15:1027–1028
Goldsmith TH, Collins JS, Licht S (1984) The cone oil droplets of avian retinas. Vis Res 24(11):1661–1671
Hart NS (2001) The visual ecology of avian photoreceptors. Prog Retin Eye Res 20(5):675–703
Hart NS, Vorobyev M (2005) Modelling oil droplet absorption spectra and spectral sensitivities of bird cone photoreceptors. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 191(4):381–392
Hart N, Partridge J, Cuthill II (1998) Visual pigments, oil droplets and cone photoreceptor distribution in the european starling (Sturnus vulgaris). J Exp Biol 201(Pt 9):1433–1446
Hart NS, Lisney TJ, Collin SP (2006) Cone photoreceptor oil droplet pigmentation is affected by ambient light intensity. J Exp Biol 209(Pt 23):4776–4787
Hayes BP, Holden AL (1983) The distribution of centrifugal terminals in the pigeon retina. Exp Brain Res 49(2):189–197
Hirsch J (1982) Falcon visual sensitivity to grating contrast. Nature 300:57–58
Ikushima M, Watanabe M, Ito H (1986) Distribution and morphology of retinal ganglion cells in the Japanese quail. Brain Res 376(2):320–334
Inzunza O, Bravo H, Smith RL, Angel M (1991) Topography and morphology of retinal ganglion cells in Falconiforms: a study on predatory and carrion-eating birds. Anat Rec 229(2):271–277
Jones MP, Pierce K Jr, Ward D (2007) Avian vision: a review of form and function with special consideration to birds of prey. J Exot Pet Med 16(2):69–87
Khattab F, Khattab FI, Fares N, Zaki A (2004) Retinal photoreceptor fine structure in some reptiles. EJHM 17:167–186
Kirschfeld K (1982) Carotenoid pigments: their possible role in protecting against photooxidation in eyes and photoreceptor cells. Proc R Soc Lond B Biol Sci 216(1202):71–85
Kolb H, Jones J (1982) Light and electron microscopy of the photoreceptors in the retina of the red-eared slider, Pseudemys scripta elegans. J Comp Neurol 209(4):331–338
Lamb TD (2009) Evolution of vertebrate retinal photoreception. Philos Trans R Soc Lond B Biol 364(1531):2911–2924
Lythgoe JN (1979) The ecology of vision. Clarendon Press, Oxford
Maier EJ, Bowmaker JK (1993) Colour vision in the passeriform bird, Leiothrix lutea: correlation of visual pigment absorbance and oil droplet transmission with spectral sensitivity. J Comp Physiol A 172:295–301
Martin GR (2012) Through birds’ eyes: insights into avian sensory ecology. J Ornithol 153(1):23–48
Martin GR, Portugal SJ (2011) Differences in foraging ecology determine variation in visual fields in ibises and spoonbills (Threskiornithidae). IBIS 153(4):662–671
Meyer DB (1977) The avian eye and its adaptations. In: Crescitelli F (ed) Handbook of sensory physiology. Springer, Berlin, pp 549–612
Morgan IG, Miethke P, Li ZK (1994) Is nitric oxide a transmitter of the centrifugal projection to the avian retina? Neurosci Lett 168(1–2):5–7
Muntz WR (1972) Inert absorbing and reflecting pigments. In: Dartnall HJ (ed) Handbook of sensory physiology. Springer, Berlin, pp 529–595
Naito J, Chen Y (2004) Morphologic analysis and classification of ganglion cells of the chick retina by intracellular injection of lucifer yellow and retrograde labeling with Dil. J Comp Nuerol 469(3):360–376
Nalbach HO, Wolf-Oberhollenzer F, Remy M (1993) Exploring the image. In: Zeigler HP, Bischof HJ (eds) Vision, brain, and behavior in birds. MIT Press, Cambridge, pp 25–46
Pettigrew JE (1978) Comparison of the retinotopic organization of the visual wulst in nocturnal and diurnal raptors, with a note on the evolution of frontal vision. In: Cool SJ, Smith EL (eds) Frontiers in visual science, vol 8. Springer series in optical sciences. Springer, Berlin, pp 328–335
Rahman ML, Sugita S, Aoyama M, Sugita S (2006) Number, distribution and size of retinal ganglion cells in the jungle crow (Corvus macrorhynchos). Anat Sci Int 81(4):253–259
Rahman ML, Aoyama M, Sugita S (2007a) Regional specialization of the Tree Sparrow Passer montanus retina: ganglion cell density and oil droplet distribution. Ornithol Sci 6(2):95–105
Rahman ML, Aoyama M, Sugita S (2007b) Topography of retinal photoreceptor cells in the Jungle Crow (Corvus macrorhynchos) with emphasis on the distribution of oil droplets. Ornithol Sci 6:29–38
Reymond L (1987) Spatial visual acuity of the falcon, Falco berigora: a behavioural, optical and anatomical investigation. Vision Res 27(10):1859–1874
Rodieck RW (1973) The vertebrate retina. W. H. Freeman and Company, San Francisco
Ruggeri M, Major JC, McKeown C, Knighton RW, Puliafito CA, Jiao S (2010) Retinal structure of birds of prey revealed by ultra-high resolution spectral-domain optical coherence tomography. Invest Ophtalmol Vis Sci 51(11):5789–5795
Schraermeyer U, Heimann K (1999) Current understanding on the role of retinal pigment epithelium and its pigmentation. Pigment Cell Res 12(4):219–236
Segovia Y, García M, Gómez-Torres MJ, Mengual R (2016) Ultrastructural study of retinal development in the turtle Trachemys scripta elegans. Zoomorphology 135:205–216
Seo JH, Haam YG, Park SW, Kim DW, Jeon GS, Lee C, Hwang CH, Kim YS, Cho SS (2001) Oligodendroglia in the avian retina: immunocytochemical demonstration in the adult bird. J Neurosci Res 65(2):173–183
Tancred E (1981) The distribution and sizes of ganglion cells in the retinas of five Australian marsupials. J Comp Neurol 196(4):585–603
Uchiyama H, Aoki K, Yonezawa S, Arimura F, Ohno H (2004) Retinal target cells of the centrifugal projection from the isthmo-optic nucleus. J Comp Neurol 476(2):146–153
Uga S, Smelser (1973) Comparative study of the fine structure of retinal Müller cells in various vertebrates. Invest Ophthalmol 12(6):434–448
Vorobyev M (2003) Coloured oil droplets enhance colour discrimination. Proc Biol Sci 270(1521):1255–1261
Vorobyev M, Osorio D (1998) Receptor noise as a determinant of colour thresholds. Proc Biol Sci 265(1394):351–358
Wagner HJ (1990) Retinal structure of fishes. In: Douglas RH, Djamgoz MB (eds) The visual system of fish. Springer Netherlands, London, pp 109–157
Walls GL (1942) The vertebrate eye and its adaptive radiation. Hafner Publising Company, New York
Walls GL, Judd HD (1933) The intra-ocular colour-filters of vertebrates. Br J Ophthalmol 17(11):641–675
Weller C, Lindstrom SH, De Grip WJ, Wilson M (2009) The area centralis in the chicken retina contains efferent target amacrine cells. Vis Neurosci 26(2):249–254
Wilby D, Toomey MB, Olsson P, Frederiksen R, Cornwall MC, Oulton R, Kelber A, Corbo JC, Roberts NW (2015) Optics of cone photoreceptors in the chicken (Gallus gallus domesticus). J R Soc Interface 12(111):20150591
Won MH, Kang TC, Cho SS (2000) Glial cells in the bird retina: immunochemical detection. Microsc Res Tech 50(2):151–160
Yau KW (1994) Phototransduction mechanism in retinal rods and cones. The Friedenwald Lecture. Invest Ophthalmol Vis Sci 35(1):9–32
Young RW, Bok D (1979) Metabolism of the pigment epithelium. In: Zinn KM, Marmot MF (eds) The retinal pigment epithelium. Harvard University Press, Cambridge, pp 103–123
Young SR, Martin GR (1984) Optics of retinal oil droplets: a model of light collection and polarization detection in the avian retina. Vis Res 24(2):129–137
Acknowledgements
This research was supported by the University of Alicante VIGROB-186 and UAUSTI16-06. We thank Drs. P. María-Mojica and A. Izquierdo of the Santa Faz Wildlife Recovery Centre for helping to obtain the samples used in this study and Vanessa Pinilla for technical support. The authors are indebted to Noemí Victory Fiol for the image, as presented in Fig. 2b. Sampling was authorized by the Regional Valencian Ministry of Agriculture, Natural Environment, Climate Change, and Rural Development.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Montoyo, Y.G., García, M. & Segovia, Y. Light and electron microscopic studies on the retina of the booted eagle (Aquila pennata). Zoomorphology 137, 177–190 (2018). https://doi.org/10.1007/s00435-017-0373-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-017-0373-8