Abstract
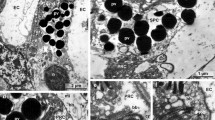
Enteropneusts or acorn worms are marine deuterostomes that have retained many plesiomorphic characters. Thus, enteropneusts are of prime interest in evolutionary comparisons between deuterostomes and protostomes. In the present study, the larval eyes of Glossobalanus marginatus were reconstructed and described based on serial sectioning for transmission electron microscopy. The everse eyes of the late Metschnikoff/early Krohn-stage tornaria larvae of G. marginatus are epidermal structures consisting of two rows of in total 13 shading pigment cells and another two rows of 13 photoreceptor cells. The pigment cells form a shallow cup with a relatively wide opening, making the cup-shaped eye optically unsuitable for picture generation. We demonstrate that the photosensitive cells possess numerous enlarged microvilli and an unmodified apical cilium. Our ultrastructural studies thus corroborate the photoreceptor cells in the eye of G. marginatus to be of a clearly rhabdomeric type. Preliminary immunohistochemical experiments support those findings by demonstrating immunopositive reaction of the tornarian eye photoreceptors with an antibody designed against rhabdomeric sea urchin photopigment (Sp-Opsin4). Observations of living animals indicate that Late Metschnikoff/early Krohn-stage tornaria larvae are negatively phototactic, probably concordant with imminent metamorphosis.



Similar content being viewed by others
References
Arendt, D., & Wittbrodt, J. (2001). Reconstructing the eyes of Urbilateria. Philosophical Transactions of the Royal Society of London B, 356, 1545–1563.
Brandenburger, J. L., Woolacott, R. M., & Eakin, R. M. (1973). Fine structure of eyespots in tornarian larvae (Phylum: Hemichordata). Cell and Tissue Research, 142, 89–102.
Cronin, T., & Porter, M. (2014). The evolution of invertebrate photopigments and photoreceptors. In D. M. Hunt, M. W. Hankins, S. P. Collin, & N. J. Marshall (Eds.), Evolution of visual and non-visual pigments (pp. 105–135). New York: Springer.
Darwin, C. (1859). The origin of species (1985th ed.). London: Penguin Book.
Eakin, R. M. (1979). Evolutionary significance of photoreceptors: in retrospect. American Zoologist, 19, 647–653.
Eakin, R. M., Martin, G. G., & Reed, C. T. (1977). Evolutionary significance of fine structure of archiannelid eyes. Zoomorphologie, 88, 1–18.
Eschscholtz, F. (1825). Bericht über die zoologische Ausbeute während der Reise von Kronstadt bis St. Peter und Paul. Oken's Isis, 16, 734–747.
Gehring, W. J. (2014). The evolution of vision. Wiley Interdisciplinary Reviews: Developmental Biology, 3, 1–40.
Meek, A. (1922). Glossobalanus marginatus, a new species of Enteropneusta from the North Sea. Quarterly Journal of Microscopical Science, 66, 579–594.
Passamaneck, Y. J., Furchheim, N., Hejnol, A., Martindale, M. Q., & Lüter, C. (2011). Ciliary photoreceptors in the cerebral eyes of a protostome larva. Evolution & Development, 2, 6.
Randel, N., Bezares-Calderón, L. A., Gühmann, M., Shahidi, R., & Jékely, G. (2013). Expression dynamics and protein localization of rhabdomeric opsins in Platynereis larvae. Integrative and Comparative Biology, 53, 7–16.
Ritter, W. E., & Davis, J. B. (1904). Studies on the ecology, morlphology, and speciology of the young of some Enteropneusta of Western North America. University of California Publications in Zoology, 1, 171–210.
Ruiz, S., & Anadón, R. (1991). The fine structure of lamellate cells in the brain of amphioxus (Branchiostoma lanceolatum, Cephalochordata). Cell and Tissue Research, 263, 591–600.
Schmidt-Ploch, U. C. (2001). Die Lochkamera. Abbildungsoptimierung. Physikalische Hintergründe. Freiburg im Breisgau: SP-Verlag.
Smith, R. S. (1984). Novel organelle associations in photoreceptors of a serpulid polychaete worm. Tissue and Cell, 16, 951–956.
Stach, T. (2014). Deuterorstome phylogeny—a morphological perspective. In W. Wägele & T. Bartolomaeus (Eds.), Deep metazoan phylogeny: The backbone of the tree of life (pp. 425–457). Berlin: De Gruyter.
Stiasny, G. (1914). Studium über die Entwicklung des Balanoglossus clavigerus Delle Chiaje. I. Die Entwicklung der Tornaria. Zeitschrift für Wissenschaftliche Zoologie, 110, 36–75. plates 34-36.
Ullrich-Lüter, E. M., Daniello, S., & Arnone, M. I. (2013). C-opsin expressing photoreceptors in echinoderms. Integrative and Comparative Biology, 53, 27–38.
Wachmann, E., Pfannenstiel, H. D., Wellmann, H., & Shelton, P. M. J. (1983). Morphogenesis of open rhabdoms in ommatidia of Leptinotarsa decemlineata and Crioceris asparagi (Coleoptera: Chrysomelidae). Zoomorphology, 103, 165–176.
Acknowledgments
We thank Maria I. Arnone for kindly providing the anti-Sp-Opsin4 antibody. Financial support by the Deutsche Forschungsgemeinschaft (DFG) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
SM1
A-G: Immunodetection of anti-Sp-Opsin4 (designed against sea urchin rhabdomeric opsin) in a tornaria larva of Glossobalanus marginatus. A: Apical view of tornaria larvae indicating area magnified in B-D; note cilia (ci) of the ciliary band. B: Higher light microscopic magnification indicated in A; arrows point to the two eyes. Rectangle demarcates area magnified in F-G. C: Immunohistochemical localization of anti-Sp-Opsin4 using confocal laser scanning microscopy showing same area as B; arrows point to the two eyes. D: Overlay of B and C. E: Higher light microscopic magnification indicated in B. F: Immunodetection of anti-Sp-Opsin4 in the respective area. G: Overlay of E and F. H-J: Immunohistochemical co-localization of antibodies raised against rhabdomeric photopigment Sp-Opsin 4 and against acetylated alpha tubulin in a tornaria larva of Glossobalanus marginatus. H: Higher light microscopic magnification showing eye depicted in SM1 E-G. I: Immunodetection of Sp-Opsin 4 using confocal laser scanning microscopy showing same area as H. J: Immunodetection of acetylated alpha tubulin using confocal laser scanning microscopy showing same area as H and I; arrowheads point to cilia in the preoral ciliary band, arrows point to neurite-like structures in the eye. ci, cilia. (GIF 196 kb)
Animation of 3D reconstruction of eye of a Late Metschnikoff/early Krohn-stage larva of Glossobalanus marginatus. Only one row of pigment cells is shown; compare to Fig. 3c. (MPG 20350 kb)
Movie showing movement of a Late Metschnikoff/early Krohn-stage larva. Fairly straight swimming movement with the apical plate forwards is propelled by the beating of the multiciliary telotroch. The two dark eyes are clearly visible. (AVI 40033 kb)
Movie showing movement of an Agassiz-stage larva. Crawling movement, with the proboscis probing the substrate is at least partially propelled by the beating of the multiciliary telotroch. Towards the end of the movie the larva speeds up due to an increase in beat rate of telotroch cilia. Contraction of muscles in the proboscis can be seen. The two dark eyes are still visible. (AVI 36849 kb)
Table SM5
(DOCX 22.7 kb)
Supplementary Methods
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Braun, K., Kaul-Strehlow, S., Ullrich-Lüter, E. et al. Structure and ultrastructure of eyes of tornaria larvae of Glossobalanus marginatus . Org Divers Evol 15, 423–428 (2015). https://doi.org/10.1007/s13127-015-0206-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-015-0206-x