Abstract
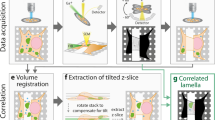
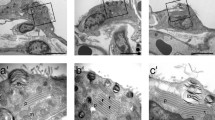
Lamellar bodies are the storage sites for lung surfactant within type II alveolar epithelial cells. The structure–function models of lamellar bodies are based on microscopic analyses of chemically fixed tissue. Despite available alternative fixation methods that are less prone to artifacts, such as cryofixation by high-pressure freezing, the nature of the lung, being mostly air filled, makes it difficult to take advantage of these improved methods. In this paper, we propose a new approach and show for the first time the ultrastructure of intracellular lamellar bodies based on cryo-electron microscopy of vitreous sections in the range of nanometer resolution. Thus, unspoiled by chemical fixation, dehydration and contrasting agents, a close to native structure is revealed. Our approach uses perfluorocarbon to substitute the air in the alveoli. Lung tissue was subsequently high-pressure frozen, cryosectioned and observed in a cryo-electron microscope. The lamellar bodies clearly show a tight lamellar morphology. The periodicity of these lamellae was 7.3 nm. Lamellar bifurcations were observed in our cryosections. The technical approach described in this paper allows the examination of the native cellular ultrastructure of the surfactant system under near in vivo conditions, and therefore opens up prospectives for scrutinizing various theories of lamellar body biogenesis, exocytosis and recycling.


Similar content being viewed by others
References
Al-Amoudi A, Chang JJ, Leforestier A, McDowall A, Salamin LM, Norlen LPO, Richter K, Blanc NS, Studer D, Dubochet J (2004a) Cryo-electron microscopy of vitreous sections. EMBO J 23:3583–3588
Al-Amoudi A, Norlen LPO, Dubochet J (2004b) Cryo-electron microscopy of vitreous sections of native biological cells and tissues. J Struct Biol 148:131–135
Al-Amoudi A, Studer D, Dubochet J (2005) Cutting artefacts and cutting process in vitreous sections for cryo-electron microscopy. J Struct Biol 150:109–121
Askin FB, Kuhn C (1971) The cellular origin of pulmonary surfactant. Lab Invest 25:260–268
Bastacky J, Lee CYC, Goerke J, Koushafar H, Yager D, Kenaga L, Speed TP, Chen Y, Clements JA (1995) Alveolar lining layer is thin and continuous—low-temperature scanning electron-microscopy of rat lung. J Appl Physiol 79:1615–1628
Braganza LF, Worcester DL (1986) Structural changes in lipid bilayers and biological membranes caused by hydrostatic pressure. Biochemistry 25:7484–7488
Clements JA (1997) Lung surfactant: a personal perspective. Annu Rev Physiol 59:1–21
Collet AJ (1979) Preservation of alveolar type-II pneumocyte lamellar bodies for electron-microscopic studies. J Histochem Cytochem 27:989–996
Costello MJ, Gulikkrzywicki T (1976) Correlated X-ray diffraction and freeze-fracture studies on membrane model systems perturbations induced by freeze-fracture preparative procedures. Biochim Biophys Acta 455:412–432
Douglas W, Redding R, Stein M (1975) The lamellar substructure of osmiophilic inclusion bodies present in rat type II alveolar pneumonocytes. Tissue Cell 7:137–142
Dubochet J, Adrian M, Chang JJ, Homo JC, Lepault J, McDowall AW, Schultz P (1988) Cryo-electron microscopy of vitrified specimens. Q Rev Biophys 21:129–228
Dubochet J, Zuber B, Eltsov M, Bouchet-Marquis C, Al-Amoudi A, Livolant F (2007) How to “read” a vitreous section. Cell Electron Microsc 79:385–406
Escaig J (1982) New instruments which facilitate rapid freezing at 83 K and 6 K. J Microsc 126:221–229
Fehrenbach H (1991) Improved preservation of phopholipid-rich multilamellar bodies in conventionally embedded mammalian lung-tissue—an electron spectroscopic study. J Microsc 162(1):91–104
Fehrenbach H (2001) Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res 2:33–46
Fehrenbach H, Ochs M (1998) Studying lung structure. In: Uhlig S, Taylor AE (eds) Methods in pulmonary research. Birkhäuser, Basel, pp 429–454
Fuhrman BP, Paczan PR, Defrancisis M (1991) Perfluorocarbon-associated gas-exchange. Crit Care Med 19:712–722
Glauert A, Lewis P (1998) Biological specimen preparation for transmission electron microscopy. In: Glauert A (ed) Practical methods in electron microscopy, vol 17. Portland Press, London, p 326
Hayat M (2000) Principles and techniques of electron microscopy: biological applications. Cambridge University Press, Cambridge, UK
Hirschl RB, Pranikoff T, Gauger P, Schreiner RJ, Dechert R, Bartlett RH (1995) Liquid ventilation in adults, children, and full-term neonates. Lancet 346:1201–1202
Ikeda H, Ichikawa A, Ichikawa M (1984) The effects of freeze-substitution media on the ultrastructure of inclusion-bodies in type-II pneumocytes of mouse lung processed by the cryofixation method. J Electron Microsc 33:242–247
Kawanami O, Lauweryns JM (1977) Observation of ultrastructure of lung-tissue with negative stain in cryo-ultramicrotomy. Acta Histochemica et Cytochemica 10:271–279
Kikkawa Y, Manabe T (1978) Freeze-fracture study of alveolar type-II cells and alveolar content in fetal rabbit lung. Anat Rec 190:627–637
Kikkawa Y, Motoyama EK, Cook CD (1965) The ultrastructure of the lungs of lamb. The relation of osmiophilic inclusions and alveolar lining layer to fetal maturation and experimentally produced respiratory distress. Am J Pathol 47:877–903
Kuhn C (1968) Cytochemistry of pulmonary alveolar epithelial cells. Am J Pathol 53:809–833
Lee CY, Bastacky J (1995) Comparative mathematical analyses of freezing in lung and solid tissue. Cryobiology 32:299–305
Liu W, Geuze HJ, Slot JW (1996) Improving structural integrity of cryosections for immunogold labeling. Histochem Cell Biol 106:41–58
Mason RJ, Williams MC (1977) Type-2 alveolar cell—defender of alveolus. Am Rev Respir Dis 115:81–91
McAteer JA, Terracio L (1984) Pulmonary type-II cell lamellar body ultrastructure preserved by rapid freezing and freeze-drying. Anat Rec 209:355–362
Muhlfeld C, Rothen-Rutishauser B, Vanhecke D, Blank F, Gehr P, Ochs M (2007) Visualization and quantitative analysis of nanoparticles in the respiratory tract by transmission electron microscopy. Part Fibre Toxicol 4(11):1–17
Niedermeyer W, Parish GR, Moor H (1977) Reactions of yeast cells to glycerol treatment—alterations to membrane structure and glycerol uptake. Protoplasma 92:177–193
Obraztsov VV, Neslund CG, Kornbrust ES, Flaim SF, Woods CM (2000) In vitro cellular effects of perfluorochemicals correlate with their lipid solubility. Am J Physiol Lung Cell Mol Phys 278:L1018–L1024
Ochs M (2010) The closer we look the more we see? Quantitative microscopic analysis of the pulmonary surfactant system. Cell Physiol Biochem 25:27–40
Ochs M, Knudsen L, Allen L, Stumbaugh A, Levitt S, Nyengaard JR, Hawgood S (2004) GM-CSF mediates alveolar epithelial type II cell changes, but not emphysema-like pathology, in SP-D-deficient mice. Am J Physiol Lung Cell Mol Physiol 287:L1333–L1341
Perez-Gil J (2008) Structure of pulmonary surfactant membranes and films: the role of proteins and lipid–protein interactions. Biochimica Et Biophysica Acta-Biomembranes 1778:1676–1695
Richter K (1994) A cryoglue to mount vitreous biological specimens for cryoultramicrotomy at 110 K. J Microsc 173:143–147
Rudiger S, Gross U, Kemnitz E (2000) Perfluorocarbons: useful tools for medicine. Eur J Med Res 5:209–216
Rudiger M, Wissel H, Ochs M, Burkhardt W, Proquitte H, Wauer RR, Stevens P, Rustow B (2003) Perfluorocarbons are taken up by isolated type II pneumocytes and influence its lipid synthesis and secretion. Crit Care Med 31:1190–1196
Rudiger M, Wendt S, Kothe L, Burkhardt W, Wauer RR, Ochs M (2007) Alterations of alveolar type II cells and intraalveolar surfactant after bronchoalveolar lavage and perfluorocarbon ventilation. An electron microscopical and stereological study in the rat lung. Respir Res 8(40):1–9
Sartori N, Richter K, Dubochet J (1993) Vitrification depth can be increased more than 10-fold by high-pressure freezing. J Microsc 172:55–61
Schulz WW, McAnalley WH, Reynolds RC (1980) Freeze-fracture study of pulmonary lamellar body membranes in solid crystal phase. J Ultrastruct Res 71:37–48
Schurch S, Goerke J, Clements JA (1976) Direct determination of surface tension in lung. Proc Natl Acad Sci USA 73:4698–4702
Semmler K, Wunderlich J, Richter W, Meyer HW (1998) High-pressure freezing causes structural alterations in phospholipid model membranes. J Microsc 190:317–327
Shaffer TH, Wolfson MR, Clark LC (1992) Liquid ventilation. Pediatr Pulmonol 14:102–109
Shimoni E, Muller M (1998) On optimizing high-pressure freezing: from heat transfer theory to a new microbiopsy device. J Microsc 192:236–247
Smith DS, Smith U, Ryan JW (1972) Freeze-fractured lamellar body membranes of rat lung great alveolar cell. Tissue Cell 4:457–468
Sorokin SP (1967) A morphologic and cytochemical study on the great alveolar cell. J Histochem Cytochem 14:884–897
Stratton CJ (1976) High-resolution ultrastructure of periodicity and architecture of lipid-retained and extracted lung multilamellar body laminations. Tissue Cell 8:713–728
Stratton CJ (1977) Periodicity and architecture of lipid retained and extracted lung surfactant and its origin from multilamellar bodies. Tissue Cell 9:301–316
Studer D, Michel M, Wohlwend M, Hunziker EB, Buschmann MD (1995) Vitrification of articular cartilage by high-pressure freezing. J Microsc 179:321–332
Studer D, Graber W, Al-Amoudi A, Eggli P (2001) A new approach for cryofixation by high-pressure freezing. J Microsc 203:285–294
Takemura T, Fukuda Y, Harrison M, Ferrans VJ (1987) Ultrastructural, histochemical, and freeze-fracture evaluation of multilamellated structures in human pulmonary alveolar proteinosis. Am J Anat 179:258–268
Terracio L, Bankston PW, McAteer JA (1981) Ultrastructural observations on tissues processed by a quick-freezing, rapid-drying method—comparison with conventional specimen preparation. Cryobiology 18:55–71
Untersee P, Gil J, Weibel ER (1971) Visualization of extracellular lining layer of lung alveoli by freeze-etching. Respir Physiol 13:171–185
Vanhecke D, Graber W, Herrmann G, Al-Amoudi A, Eggli P, Studer D (2003) A rapid microbiopsy system to improve the preservation of biological samples prior to high-pressure freezing. J Microsc 212:3–12
Vanhecke D, Eggli P, Graber W, Studer D (2006) A new microbiopsy system enables rapid preparation of tissue for high-pressure freezing. Methods Mol Med, pp 463–477
Varga T, Wilkinson AP, Angel RJ (2003) Fluorinert as a pressure-transmitting medium for high-pressure diffraction studies. Rev Sci Instrum 74:4564–4566
Walker SR, Williams MC, Benson B (1986) Immunocytochemical localization of the major surfactant apoproteins in type-II cells, clara cells, and alveolar macrophages of rat lung. J Histochem Cytochem 34:1137–1148
Weaver TE, Na CL, Stahlman M (2002) Biogenesis of lamellar bodies, lysosome-related organelles involved in storage and secretion of pulmonary surfactant. Seminars Cell Dev Biol 13:263–270
Weibel ER, Kistler GS, Tondury G (1966) A stereologic electron microscope study of tubular myelin figures in alveolar fluids of rat lungs. Zeitschrift für Zellforschung und Mikroskopische Anatomie 69:418–427
Williams MC (1978) Freeze-fracture studies of tubular myelin and lamellar bodies in fetal and adult rat lungs. J Ultrastruct Res 64:352–361
Willison M, Rowe A (1980) Replica, shadowing and freeze-etching techniques. Freeze-fracturing and freeze-etching. North-Holland Publishing Company, Amsterdam, p 301
Wolf GK, Sheeran P, Heitz D, Thompson JE, Arnold JH (2008) Gas exchange and lung mechanics during high frequency ventilation in the perflubron-treated lung. Pediatr Crit Care Med 9:641–646
Wright JR (1997) Immunomodulatory functions of surfactant. Physiol Rev 77:931–962
Wright JR (2005) Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 5:58–68
Wright TW, Notter RH, Wang ZD, Harmsen AG, Gigliotti F (2001) Pulmonary inflammation disrupts surfactant function during Pneumocystis carinii pneumonia. Infect Immun 69:758–764
Yang CL, Terada N, Ohno N, Fujii Y, Ohno S (2006) Morphological analysis of lamellar structures in mouse type II pneumocytes by quick-freezing and freeze-drying with osmium tetroxide vapor-fixation. Med Mol Morphol 39:88–96
Zuber B, Nikonenko I, Klauser P, Muller D, Dubochet J (2005) The mammalian central nervous synaptic cleft contains a high density of periodically organized complexes. Proc Natl Acad Sci USA 102:19192–19197
Acknowledgments
This study has been supported in part by grants from the Swiss National Science Foundation (SNF 3100AO-116417) to M.O. and (SNF 31003A-118394) to D.S. The experiments were performed according to the 3R principle and were approved by the cantonal administration of Bern, Switzerland for animal testing (38/09) to D.V.
Author information
Authors and Affiliations
Corresponding author
Additional information
D. Vanhecke and G. Herrmann contributed equally to this study.
Rights and permissions
About this article
Cite this article
Vanhecke, D., Herrmann, G., Graber, W. et al. Lamellar body ultrastructure revisited: high-pressure freezing and cryo-electron microscopy of vitreous sections. Histochem Cell Biol 134, 319–326 (2010). https://doi.org/10.1007/s00418-010-0736-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-010-0736-4