Abstract
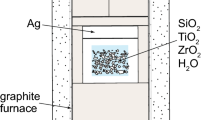
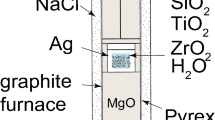
Several studies have reported the P–T dependencies of Ti-in-quartz solubility, and there is close agreement among three of the four experimental calibrations. New experiments were conducted in the present study to identify potential experimental disequilibrium, and to determine which Ti-in-quartz solubility calibration is most accurate. Crystals of quartz, rutile and zircon were grown from SiO2-, TiO2-, and ZrSiO4-saturated aqueous fluids in an initial synthesis experiment at 925 °C and 10 kbar in a piston-cylinder apparatus. A range of quartz crystal sizes was produced in this experiment; both large and small examples were analyzed by electron microprobe to determine whether Ti concentrations are correlated with crystal size. Cathodoluminescence images and EPMA measurements show that intercrystalline and intracrystalline variations in Ti concentrations are remarkably small regardless of crystal size. The average Ti-in-quartz concentration from the synthesis experiment is 392 ± 1 ppmw Ti, which is within 95 % confidence interval of data from the 10 kbar isobar of Wark and Watson (Contrib Mineral Petrol 152:743–754, 2006) and Thomas et al. (Contrib Mineral Petrol 160:743–759, 2010). As a cross-check on the Ti-in-quartz calibration, we also measured the concentration of Zr in rutile from the synthesis experiment. The average Zr-in-rutile concentration is 4337 ± 32 ppmw Zr, which is also within the 95 % confidence interval of the Zr-in-rutile solubility calibration of Ferry and Watson (Contrib Mineral Petrol 154:429–437, 2007). The P–T dependencies of Ti solubility in quartz and Zr solubility in rutile were applied as a thermobarometer to the experimental sample. The average Ti-in-quartz isopleth calculated from the calibration of Thomas et al. (Contrib Mineral Petrol 160:743–759, 2010) and the average Zr-in-rutile isopleth calculated from the calibration of Tomkins et al. (J Metamorph Geol 25:703–713, 2007) cross at 9.5 kbar and 920 °C, which is in excellent agreement with the P–T conditions of the synthesis experiment. Separates of the high-Ti quartz from the initial synthesis experiment described above were used as starting material in subsequent experiments at 20 kbar, at which pressure the solubility of Ti in quartz is expected to be significantly lower in the recrystallized quartz. These recrystallization experiments were conducted under wet and dry conditions at 925 °C, and under wet conditions at 850 °C. Both wet and dry recrystallization experiments produced polycrystalline quartzites. Rutile occurs as inclusions in quartz, and as individual crystals dispersed along quartz grain boundaries. Quartz that grew during the recrystallization experiments has dark cathodoluminescence indicating substantially lower Ti concentrations. The average Ti concentrations in quartz from the recrystallization experiments are within the 95 % confidence interval of a linear fit to the 20 kbar data of Thomas et al. (Contrib Mineral Petrol 160:743–759, 2010). Collectively, the results from the synthesis and recrystallization experiments confirm that the Ti-in-quartz concentrations used to calibrate the P–T dependencies of Ti-in-quartz solubility in Thomas et al.’s (Contrib Mineral Petrol 160:743–759, 2010) calibration represent the equilibrium concentrations of Ti in quartz.








Similar content being viewed by others
References
Antignano A, Manning CE (2008) Rutile solubility in H2O, H2O–SiO2, and H2O–NaAlSi3O8 fluid at 0.7–2.0 GPa and 700–1000°C: implications for mobility of nominally insoluble elements. Chem Geol 255:283–293
Ashley KT, Webb LE, Spear FS, Thomas JB (2013) P-T-D histories from quartz: a case study of the application of the TitaniQ thermobarometer to progressive fabric development in metapelites. Geochem Geophys Geosyst 14:3821–3843. doi:10.1002/ggge.20237
Ashley KT, Caddick MJ, Steele-MacInnis M, Bodnar RJ, Dragovic B (2014) A detailed history of deep burial and exhumation recorded by quartz inclusions in garnet. Geochem Geophys Geosyst 15:350–360
Audétat A (2013) Origin of Ti-rich rims in quartz phenocrysts from the Upper Bandelier Tuff and the Tunnel Spring Tuff, southwestern USA. Chem Geol 360–361:99–104
Behr WM, Platt JP (2011) A naturally constrained stress profile through the middle crust in an extensional terrane. Earth Planet Sci Lett 303:181–192
Breiter K, Ackerman L, Svojtka M, Müller A (2013) Behavior of trace elements in quartz from plutons of different geochemical signature: a case study from the Bohemian Massif, Czech Republic. Lithos 175–176:54–67
Cheng L, Fenter P, Nagy K, Schlegel M, Sturchio NC (2001) Molecular scale density oscillations in water adjacent to a mica surface. Phys Rev Lett 87:56103–1–56103-4
Cherniak DJ, Watson EB, Wark DA (2007) Ti diffusion in quartz. Chem Geol 236:65–74
Downs RT (2006) The RRUFF Project: an integrated study of the chemistry, crystallography, Raman and infrared spectroscopy of minerals. Program and Abstracts of the 19th General Meeting of the International Mineralogical Association in Kobe, Japan. O03-13
Eng PJ, Trainor TP, Brown GE (2000) Structure of the hydrated alpha-Al2O3 surface. Science 288:1029–1033
Fenter P, Teng H, Geissbuhler P, Nagy K, Sturchio NC (2000) Atomic scale structure of orthoclase (001)-water interface measured with X-ray reflectivity. Geochim Cosmochim Acta 64:3663–3673
Fenter P, McBride MT, Srajer G, Sturchio NC, Bosbach D (2001) Structure of barite (100)- and (210)-water interfaces. J Phys Chem B 105:8112–8119
Ferry JM, Watson EB (2007) New thermodynamic models and revised calibrations for the Ti-in-zircon and Zr-in-rutile thermometers. Contrib Mineral Petrol 154:429–437
Ghiorso MS, Gualda GAR (2013) A method for estimating the activity of titania in magmatic liquids from the compositions of coexisting rhombohedral and cubic iron–titanium oxides. Contrib Mineral Petrol 165:73–81
Grujic G, Stipp M, Wooden JL (2011) Thermometry of quartz mylonites: importance of dynamic recrystallization on Ti-in-quartz reequilibration. Geochem Geophys Geosyst. doi:10.1029/2010GC003368
Hays RS, Evans B (1987) Chemically induced grain boundary migration in calcite: temperature dependence, phenomenology, and possible applications to geologic systems. Contrib Mineral Petrol 97:127–141
Hillert M, Purdy GR (1978) Chemically induced grain boundary migration. Acta Metall 26:333–340
Huang R, Audétat A (2012) The titanium-in-quartz (TitaniQ) thermobarometer: a critical examination and re-calibration. Geochim Cosmochim Acta 84:75–89
Kidder S, Avouac J-P, Chan Y-C (2013) Application of titanium-in-quartz thermobarometry to greenschist facies veins and recrystallized quartzites in the Hsüehshan range, Taiwan. Solid Earth 4:1–21
Kohn MJ (2014) “Geoba-Raman-try”: calibration of spectroscopic barometers for mineral inclusions. Earth Planet Sci Lett 388:187–196
Kohn MJ, Northrup CJ (2009) Taking mylonites’ temperatures. Geology 37:47–50
Kularatne K, Audétat A (2014) Rutile solubility in hydrous rhyolite melts at 750–900°C and 2 kbar, with application to titanium-in-quartz (TitaniQ). Geochim Cosmochim Acta 125:196–209
Lanzillo NA, Watson EB, Thomas JB, Nayak S, Curioni A (2014) Near-surface controls on the composition of growing crystals: car-parrinello molecular dynamics (CPMD) simulations of Ti4+ energetics and diffusion in alpha quartz. Geochim Cosmochim Acta 131:33–46
Maldener J, Rauch F, Gavranic M, Beran A (2001) OH absorption coefficients of rutile and cassiterite deduced from nuclear reaction analysis and FTIR spectroscopy. Mineral Petrol 71:21–29
Nachlas WO, Whitney DL, Teyssier C, Bagley B, Mulch A (2014) Titanium concentration in quartz as a record of multiple deformation mechanisms in an extensional shear zone. Geochem Geophys Geosyst 15:1374–1397. doi:10.1002/2013GC005200
Negrini M, Stunitz H, Berger A, Morales LFG (2014) The effect of deformation on the TitaniQ geothermobarometer: an experimental study. Contrib Mineral Petrol 167:982. doi:10.1007/s00410-014-0982-x
Ostapenko GT, Gamarnik MY, Gorogotskaya LI, Kuznetsov GV, Tarashchan AN, Timoshkova LP (1987) Isomorphism of titanium substitution for silicon in quartz: experimental data. Mineral Zh 9:30–40
Ostapenko GT, Tarashchan AN, Mitsyuk BM (2007) Rutile-quartz geothermobarometer. Geochem Int 45:506–550
Riggi V, Thomas JB, Tailby N, Watson EB (2014) Aluminum substitution in quartz and its effect on dissolved H2O. American Geophysical Union Fall Meeting abstract V41A-4781
Schlegel ML, Nagy KL, Fenter P, Sturchio NC (2002) Structures of quartz (1010)- and (1010)-water interfaces determined by X-ray reflectivity and atomic force microscopy of natural growth faces. Geochim Cosmochim Acta 66:3037–3054
Spear FS, Wark DA (2009) Cathodoluminescence imaging and titanium thermometry in metamorphic quartz. J Metamorph Geol 27:187–205
Spear FS, Ashley KT, Webb LE, Thomas JB (2012) Ti diffusion in quartz inclusions: implications for metamorphic timescales. Contrib Miner Petrol 164:977–986
Spear FS, Thomas JB, Hallett BW (2014) Overstepping the garnet isograd: a comparison of QuiG barometry and thermodynamic modeling. Contrib Mineral Petrol 168:1059. doi:10.1007/s00410-014-1059-6
Thomas JB, Watson EB (2012) Application of the Ti-in-quartz thermobarometer to rutile-free systems. Reply to: a comment on: ‘TitaniQ under pressure: the effect of pressure and temperature on the solubility of Ti in quartz’ by Thomas et al. Contrib Mineral Petrol 164:369–374
Thomas S-M, Koch-Müller M, Reichart P, Rhede D, Thomas R, Wirth R, Matsyuk S (2009) IR calibrations for water determination in olivine, r-GeO2, and SiO2 polymorphs. Phys Chem Mineral 36:489–509
Thomas JB, Watson EB, Spear FS, Shemella FS, Nayak SK, Lanzirotti A (2010) TitaniQ under pressure: the effect of pressure and temperature on the solubility of Ti in quartz. Contrib Mineral Petrol 160:743–759
Tomkins HS, Powell R, Ellis DJ (2007) The pressure dependence of the zirconium-in-rutile thermometer. J Metamorph Geol 25:703–713
Wark DA, Watson EB (2006) The TitaniQ: a titanium-in-quartz geothermometer. Contrib Mineral Petrol 152:743–754
Wark DA, Hildreth W, Spear FS, Cherniak DJ, Watson EB (2007) Pre-eruption recharge of the Bishop magma system. Geology 35:235–238
Watson EB (1996) Surface enrichment and trace-element uptake during crystal growth. Geochim Cosmochim Acta 60:5013–5020
Watson EB (2004) A conceptual model for near-surface kinetic controls on the trace-element and stable isotope composition of abiogenic calcite crystals. Geochim Cosmochim Acta 68:1473–1488
Watson EB, Liang Y (1995) A simple model for sector zoning in slowly-grown crystals: implications for growth rate and lattice diffusion, with emphasis on accessory minerals in crustal rocks. Am Mineral 80:1170–1187
Watson EB, Müller T (2009) Non-equilibrium isotopic and elemental fractionation during diffusion-controlled crystal growth under static and dynamic conditions. Chem Geol 267:111–124
Watson EB, Wark DA, Price JD, Van Orman JA (2002) Mapping the thermal structure of solid-media pressure assemblies. Contrib Mineral Petrol 142:640–652
Watson EB, Wark DA, Thomas JB (2006) Crystallization thermometers for zircon and rutile. Contrib Mineral Petrol 151:413–433
Wilson CJN, Seward TM, Allan ASR, Charlier BLA, Bello L (2012) A comment on: ‘TitaniQ under pressure: the effect of pressure and temperature on the solubility of Ti in quartz’, by Jay B. Thomas, E. Bruce Watson, Frank S. Spear, Philip T. Shemella, Saroj K. Nayak and Antonio Lanzirotti. Contrib Miner Petrol 164:359–368. doi:10.1007/s00410-012-0757-1
Acknowledgments
This work was supported by the Earth Sciences Division of the National Science Foundation through grant numbers EAR–1220295 to JBT, and EAR–0948987 to JBT and EBW. Comments by handling editor M. Ghiorso and reviews by J. Mavrogenes, J. Gardner, and G. Gualda helped improve the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Mark S Ghiorso.
Electronic supplementary material
Below is the link to the electronic supplementary material.
410_2015_1120_MOESM1_ESM.eps
Supplementary Figure A. Unpolarized transmission infrared spectra of the OH region of a (001) section of quartz (a) and a (100) section of rutile (b) from the synthesis experiment QTiP-43. The absorption bands are from trace hydrogen incorporated as hydroxyl in the crystals. (EPS 1890 kb)
Rights and permissions
About this article
Cite this article
Thomas, J.B., Watson, E.B., Spear, F.S. et al. TitaniQ recrystallized: experimental confirmation of the original Ti-in-quartz calibrations. Contrib Mineral Petrol 169, 27 (2015). https://doi.org/10.1007/s00410-015-1120-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00410-015-1120-0