Abstract
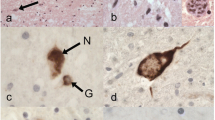
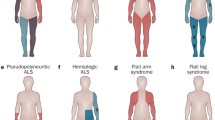
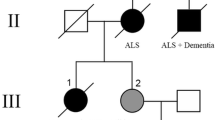
Mutations in the gene encoding the fused in sarcoma (FUS) protein are responsible for ~3% of familial amyotrophic lateral sclerosis (ALS) and <1% of sporadic ALS (ALS-FUS). Descriptions of the associated neuropathology are few and largely restricted to individual case reports. To better define the neuropathology associated with FUS mutations, we have undertaken a detailed comparative analysis of six cases of ALS-FUS that include sporadic and familial cases, with both juvenile and adult onset, and with four different FUS mutations. We found significant pathological heterogeneity among our cases, with two distinct patterns that correlated with the disease severity and the specific mutation. Frequent basophilic inclusions and round FUS-immunoreactive (FUS-ir) neuronal cytoplasmic inclusions (NCI) were a consistent feature of our early-onset cases, including two with the p.P525L mutation. In contrast, our late-onset cases that included two with the p.R521C mutation had tangle-like NCI and numerous FUS-ir glial cytoplasmic inclusions. Double-labeling experiments demonstrated that many of the glial inclusions were in oligodendrocytes. Comparison with the neuropathology of cases of frontotemporal lobar degeneration with FUS-ir pathology showed significant differences and suggests that FUS mutations are associated with a distinct pathobiology.





Similar content being viewed by others
Notes
We have found that newer batches of Sigma anti-FUS, obtained following completion of this study, provide similar results when used at concentrations of 1:500–1:2,000.
References
Aizawa H, Kimura T, Hashimoto K et al (2000) Basophilic cytoplasmic inclusions in a case of sporadic juvenile amyotrophic lateral sclerosis. J Neurol Sci 176:109–113
Andersson MK, Stahlberg A, Arvidsson Y et al (2008) The multifunctional FUS, EWS, and TAF15 proto-oncoproteins show cell type-specific expression patterns and involvement in cell spreading and stress response. BMC Cell Biol 9:37
Baumer D, Hilton D, Paine AML et al (2010) Juvenile ALS with basophilic inclusions is a FUS proteinopathy with FUS mutations. Neurology 75:611–618
Blair IP, Williams KL, Warrich ST et al (2010) FUS mutations in amyotrophic lateral sclerosis: clinical, pathological, neurophysiological and genetic analysis. J Neurol Neurosurg Psychiatry 81:639–645
Dormann D, Rodde R, Edbauer D et al (2010) ALS-associated fused in sarcoma (FUS) mutations disrupt transportin-mediated nuclear import. EMBO J 29:2841–2857
Fujita Y, Fujita S, Takatama M, Ikeda M, Okamoto K (2011) Numerous FUS-positive inclusions in an elderly woman with motor neuron disease. Neuropathology 31:170–176
Fujita K, Ito H, Nakano S et al (2008) Immunohistochemical identification of messenger RNA-related proteins in basophilic inclusions of adult-onset atypical motor neuron disease. Acta Neuropathol 116:439–445
Groen EJN, van Es MA, van Vught PWJ et al (2010) FUS mutation sin familial amyotrophic lateral sclerosis in the Netherlands. Arch Neurol 67:224–230
Hamada K, Fukazawa T, Yanagihara T et al (1995) Dementia with ALS features and diffuse Pick body-like inclusions (atypical Pick’s disease?). Clin Neuropathol 14:1–6
Hewitt C, Kirby J, Highley R et al (2010) Novel FUS/TLS mutations and pathology in familial and sporadic amyotrphic lateral sclerosis. Arch Neurol 67:455–461
Huang EJ, Zhang J, Geser F et al (2010) Extensive FUS-immunoreactive pathology in juvenile amyotrophic lateral sclerosis with basophilic inclusions. Brain Pathol 20:1069–1076
Ishihara K, Araki S, Ihori N et al (2006) An autopsy case of frontotemporal dementia with severe dysarthria and motor neuron disease showing numerous basophilic inclusions. Neuropathology 26:447–454
Ito D, Seki M, Tsunoda Y, Uchiyama H, Suzuki N (2011) Nuclear transport impairment of amyotrophic lateral sclerosis-linked mutations in FUS/TLS. Ann Neurol 69:152–162
Kino Y, Washizu C, Aquilanti E et al (2011) Intracellular localization and splicing regulation of FUS/TLS are variably affected by amyotrophic lateral sclerosis-linked mutations. Nucleic Acids Res 39:2781–2798
Kobayashi Z, Tsuchiya K, Arai T et al (2010) Occurrence of basophilic inclusions and FUS-immunoreactive neuronal and glial inclusions in a case of familial amyotrophic lateral sclerosis. J Neurol Sci 293:6–11
Kusaka H, Matsumoto S, Imai T (1990) An adult-onset case of sporadic motor neuron disease with basophilic inclusions. Acta Neuropathol 80:660–665
Kusaka H, Matsumoto S, Imai T (1993) Adult-onset motor neuron disease with basophilic intraneuronal inclusion bodies. Clin Neuropathol 12:215–218
Kwiatkowski TJ, Bosco DA, LeClerc AL et al (2009) Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323:1205–1208
Mackenzie IRA, Foti D, Woulfe J, Hurwitz TA (2008) Atypical frontotemporal lobar degeneration with ubiquitin-positive, TDP-43-negative neuronal inclusions. Brain 131:1282–1293
Mackenzie IRA, Munoz DG, Kusaka H et al (2011) Distinct pathological subtypes of FTLD-FUS. Acta Neuropathol 121:207–218
Mackenzie IRA, Neumann M, Bigio EH et al (2010) Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 119:1–4
Mackenzie IRA, Rademakers R, Neumann M (2010) TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol 9:995–1007
Matsuoka T, Fujii N, Kondo A et al (2011) An autopsied case of sporadic adult-onset amyotrophic lateral sclerosis with FUS-positive basophilic inclusions. Neuropathology 31:71–76
Munoz-Garcia D, Ludwin SK (1984) Classic and generalized variants of Pick’s disease: a clinicopathological, ultrastructural, and immunocytochemical comparative study. Ann Neurol 16:467–480
Munoz DG, Neumann M, Kusaka H et al (2009) FUS pathology in basophilic inclusion body disease. Acta Neuropathol 118:617–627
Nelson JS, Prensky AL (1972) Sporadic juvenile amyotrophic lateral sclerosis. A clinicopathological study of a case with neuronal cytoplasmic inclusions containing RNA. Arch Neurol 27:300–306
Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, Mackenzie IRA (2009) Frontotemporal lobar degeneration with FUS pathology. Brain 132:2922–2931
Neumann M, Roeber S, Kretzschmar HA, Rademakers R, Baker M, Mackenzie IRA (2009) Abundant FUS pathology in neuronal intermediate filament inclusion disease. Acta Neuropathol 118:605–616
Rademakers R, Stewart H, DeJesus-Hernandez M et al (2010) FUS gene mutations in familial and sporadic amyotrophic lateral sclerosis. Muscle Nerve 42:170–176
Robertson J, Bilbao J, Zinman L et al. (2011) A novel double mutation in FUS gene causing sporadic ALS. Neurobiol Aging 32:553.e27–553.e30
Roeber S, Mackenzie IR, Kretzschmar HA, Neumann M (2008) TDP-43-negative FTLD-U is a significant new clinico-pathological subtype of FTLD. Acta Neuropathol 116:147–157
Rohrer JD, Lashley T, Holton J et al. (2010) The clinical and neuroanatomical phenotype of FUS associated frontotemporal lobar degeneration. J Neurol Neursurg Psychiatry. doi:10.1136/jnnp.2010.214437
Sasaki S, Toi S, Shirata A et al (2001) Immunohistochemical and ultrastructural study of basophilic inclusions in adult-onset motor neuron disease. Acta Neuropathol 102:200–206
Suzuki N, Aoki M, Warita H et al (2010) FALS with FUS mutation in Japan, with early onset, rapid progress and basophilic inclusions. J Hum Genet 55:252–254
Tateishi T, Hokonohara T, Yamasaki R et al (2010) Multiple system degeneration with basophilic inclusions in Japanese ALS patients with FUS mutation. Acta Neuropathol 119:355–364
Tsuchiya K, Matsunaga T, Aoki M et al (2001) Familial amyotrophic lateral sclerosis with posterior column degeneration and basophilic inclusion bodies: a clinical, genetic and pathological study. Clin Neuropathol 20:53–59
Urwin H, Josephs KA, Rohrer JD et al (2010) FUS pathology defines the majority of tau-and TDP-43-negative frontotemporal lobar degeneration. Acta Neuropathol 120:33–41
Vance C, Rogelj B, Hortobagyi T et al (2009) Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323:1208–1211
Yamamoto-Watanabe Y, Watanabe M, Okamoto K et al (2010) A Japanese ALS6 family with mutation R521C in the FUS/TLS gene: a clinical, pathological and genetic report. J Neurol Sci 296:59–63
Yang S, Warraich ST, Nicholson GA, Blair IP (2010) Fused in sarcoma/translocated in liposarcoma: a multifunctional DNA/RNA binding protein. Int J Biochem Cell Biol 42:1408–1411
Zinszner H, Sok J, Immanuel D, Yin Y, Ron D (1997) TLS (FUS) binds RNA in vivo and engages in nucleo-cytoplasmic shuttling. J Cell Sci 110:1741–1750
Acknowledgments
We thank Margaret Luk and Mareike Schroff for their excellent technical assistance. This work was supported by grants from the Canadian Institutes of Health Research (Grant Number 74580, IM), the Pacific Alzheimer Research Foundation (IM), the NIHR Oxford Biomedical Research Centre (OA), the NIH/NIA R01 AG26251 (RR), the ALS Association (RR), the Swiss National Science Foundation (Grant Number 31003A-132864, MN), the Stavros-Niarchos Foundation (MN) and the Synapsis Foundation (MN).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mackenzie, I.R.A., Ansorge, O., Strong, M. et al. Pathological heterogeneity in amyotrophic lateral sclerosis with FUS mutations: two distinct patterns correlating with disease severity and mutation. Acta Neuropathol 122, 87–98 (2011). https://doi.org/10.1007/s00401-011-0838-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-011-0838-7