Abstract
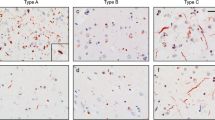
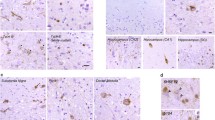
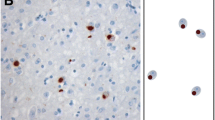
Most cases of frontotemporal lobar degeneration (FTLD) are characterized by abnormal intracellular accumulation of either tau or TDP-43 protein. However, in ~10% of cases, composed of a heterogenous collection of uncommon disorders, the molecular basis remains to be uncertain. We recently discovered that the pathological changes in several tau/TDP-43-negative FTLD subtypes are immunoreactive (ir) for the fused in sarcoma (FUS) protein. In this study, we directly compared the pattern of FUS-ir pathology in cases of atypical FTLD-U (aFTLD-U, N = 10), neuronal intermediate filament inclusion disease (NIFID, N = 5) and basophilic inclusion body disease (BIBD, N = 8), to determine whether these are discrete entities or represent a pathological continuum. All cases had FUS-ir pathology in the cerebral neocortex, hippocampus and a similar wide range of subcortical regions. Although there was significant overlap, each group showed specific differences that distinguished them from the others. Cases of aFTLD-U consistently had less pathology in subcortical regions. In addition, the neuronal inclusions in aFTLD-U usually had a uniform, round shape, whereas NIFID and BIBD were characterized by a variety of inclusion morphologies. In all cases of aFTLD-U and NIFID, vermiform neuronal intranuclear inclusions (NII) were readily identified in the hippocampus and neocortex. In contrast, only two cases of BIBD had very rare NII in a single subcortical region. These findings support aFTLD-U, NIFID and BIBD as representing closely related, but distinct entities that share a common molecular pathogenesis. Although cases with overlapping pathology may exist, we recommend retaining the terms aFTLD-U, NIFID and BIBD for specific FTLD-FUS subtypes.



Similar content being viewed by others
References
Andersson MK, Stahlberg A, Arvidsson Y et al (2008) The multifunctional FUS, EWS, and TAF15 proto-oncoproteins show cell type-specific expression patterns and involvement in cell spreading and stress response. BMC Cell Biol 9:37
Arai T, Hasegawa M, Akiyama H et al (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351:602–611
Baumer D, Hilton D, Paine AML et al (2010) Juvenile ALS with basophilic inclusions is a FUS proteinopathy with FUS mutations. Neurology 75:611–618
Behring B, Beuche W, Kretzschmar HA (1998) Progressive dementia with parkinsonism in corticobasal degeneration and brainstem degeneration with neuronal inclusions. Neurology 51:285–288
Bigio EH, Lipton AM, White CL, Dickson DW, Hirano A (2003) Frontotemporal and motor neurone degeneration with neurofilament inclusion bodies: additional evidence for overlap between FTD and ALS. Neuropathol Appl Neurobiol 29:239–253
Blair IP, Williams KL, Warrich ST et al (2010) FUS mutations in amyotrophic lateral sclerosis: clinical, pathological, neurophysiological and genetic analysis. J Neurol Neurosurg Psychiatry 81:639–645
Cairns NJ, Grossman M, Arnold SE et al (2004) Clinical and neuropathological variation in neuronal intermediate filament inclusion disease. Neurology 63:1376–1384
Cairns NJ, Perry RH, Jaros E et al (2003) Patients with a novel neurofilamentopathy: dementia with neurofilament inclusions. Neurosci Lett 341:177–180
Cairns NJ, Zhukareva V, Uryu K et al (2004) α-Internexin is present in the pathological inclusions of neuronal intermediate filament inclusion disease. Am J Pathol 164:2153–2161
Davidson Y, Kelley T, Mackenzie IRA et al (2007) Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol 113:521–533
Fujii R, Okabe S, Urushido T et al (2005) The RNA binding protein TLS is translocated to dendritic spines by mGluR5 activation and regulates spine morphology. Curr Biol 15:587–593
Fujii R, Takumi T (2005) TLS facilitates transport of mRNA encoding an actin-stabilizing protein to dendritic spines. J Cell Sci 118:5755–5765
Fujita Y, Fujita S, Takatama M, Ikeda M, Okamoto K (2010) Numerous FUS-positive inclusions in an elderly woman with motor neuron disease. Neuropathology. doi:10.1111/j.1440-1789.2010.01146.x
Groen EJN, van ES MA, van Vught PWJ et al (2010) FUS mutations in familial amyotrophic lateral sclerosis in the Netherlands. Arch Neurol 67:224–230
Hamada K, Fukazawa T, Yanagihara T et al (1995) Dementia with ALS features and diffuse Pick body-like inclusions (atypical Pick’s disease?). Clin Neuropathol 14:1–6
Hewitt C, Kirby J, Highley R et al (2010) Novel FUS/TLS mutations and pathology in familial and sporadic amyotrophic lateral sclerosis. Arch Neurol 67:455–461
Huang EJ, Zhang J, Geser F et al (2010) Extensive FUS-immunoreactive pathology in juvenile amyotrophic lateral sclerosis with basophilic inclusions. Brain Pathol 20:1069–1076
Ishihara K, Araki S, Ihori N et al (2006) An autopsy case of frontotemporal dementia with severe dysarthria and motor neuron disease showing numerous basophilic inclusions. Neuopathology 26:447–454
Josephs KA, Holton JL, Rossor MN et al (2003) Neurofilament inclusion body disease: a new proteinopathy? Brain 126:2291–2303
Josephs KA, Holton JL, Rossor MN et al (2004) Frontotemporal lobar degeneration and ubiquitin immunohistochemistry. Neuropathol Appl Neurobiol 30:369–373
Josephs KA, Lin WL, Ahmed Z, Stroh DA, Graff-Radford NR, Dickson DW (2008) Frontotemporal lobar degeneration with ubiquitin-positive, but TDP-43-negative inclusions. Acta Neuropathol 116:159–167
Josephs KA, Uchikado H, McComb RD et al (2005) Extending the clinicopathological spectrum of neurofilament inclusion disease. Acta Neuropathol 109:427–432
Kobayashi Z, Tsuchiya K, Arai T et al (2010) Occurence of basophilic inclusions and FUS-immunoreactive neuronal and glial inclusions in a case of familial amyotrophic lateral sclerosis. J Neurol Sci 293:6–11
Kusaka H, Matsumoto S, Imai T (1990) An adult-onset case of sporadic motor neuron disease with basophilic inclusions. Acta Neuropathol 80:660–665
Kusaka H, Matsumoto S, Imai T (1993) Adult-onset motor neuron disease with basophilic intraneuronal inclusion bodies. Clin Neuopathol 12:215–218
Kwiatkowski TJ, Bosco DA, LeClerc AL et al (2009) Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323:1205–1208
Lagier-Tourenne C, Cleveland DW (2009) Rethinking ALS: the FUS about TDP-43. Cell 136:1001–1004
Loy CT, McCusker E, Kril JJ et al (2010) Very early-onset frontotemporal dementia with no family history predicts underlying fused in sarcoma pathology. Brain. doi:10.1093/brain/awq186
Mackenzie IR, Feldman H (2004) Neurofilament inclusion body disease with early onset frontotemporal dementia and primary lateral sclerosis. Clin Neuropathol 23:183–193
Mackenzie IRA, Foti D, Woulfe J, Hurwitz TA (2008) Atypical frontotemporal lobar degeneration with ubiquitin-positive, TDP-43-negative neuronal inclusions. Brain 131:1282–1293
Mackenzie IR, Neumann M, Bigio EH et al (2009) Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol 117:15–18
Mackenzie IRA, Neumann M, Bigio EH et al (2010) Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 119:1–4
Mackenzie IRA, Rademakers R, Neumann M (2010) TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol 9:995–1007
Mackenzie IRA, Shi J, Shaw CL et al (2006) Dementia lacking distinctive histology (DLDH) revisited. Acta Neuropathol 112:551–559
Matsuoka T, Fujii N, Kondo A et al (2010) An autopsied case of sporadic adult-onset amyotrophic lateral sclerosis with FUS-positive basophilic inclusions. Neuropathology. doi:10.1111/j.1440-1789.2010.01129.x
Molina-Porcel L, Llado A, Rey MJ et al (2008) Clinical and pathological heterogeneity of neuronal intermediate filament inclusion disease. Arch Neurol 65:272–275
Munoz-Garcia D, Ludwin SK (1984) Classic and generalized variants of Pick’s disease: a clinicopathological, ultrastructural, and immunocytochemical comparative study. Ann Neurol 16:467–480
Munoz DG, Neumann M, Kusaka H et al (2009) FUS pathology in basophilic inclusion body disease. Acta Neuropathol 118:617–627
Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, Mackenzie IRA (2009) Frontotemporal lobar degeneration with FUS pathology. Brain 132:2922–2931
Neumann M, Roeber S, Kretzschmar HA, Rademakers R, Baker M, Mackenzie IRA (2009) Abundant FUS pathology in neuronal intermediate filament inclusion disease. Acta Neuropathol 118:605–616
Neumann M, Sampathu DM, Kwong LK et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133
Neumann M, Tolnay M, Mackenzie IRA (2009) The molecular basis of frontotemporal dementia. Exp Rev Mol Med 11:e23
Rademakers R, Stewart H, DeJesus-Hernandez M et al (2010) FUS gene mutations in familial and sporadic amyotrophic lateral sclerosis. Muscle Nerve 42:170–176
Riggi N, Cironi L, Suva ML, Stamenkovic I (2007) Sarcomas: genetics, signalling, and cellular origins. Part I: The fellowship of TET. J Pathol 213:4–20
Robertson J, Bilbao J, Zinman L et al (2010) A novel double mutation in FUS gene causing sporadic ALS. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2010.05.015
Roeber S, Bazner H, Hennerici M, Porstmann R, Kretzschmar HA (2006) Neurodegeneration with features of NIFID and ALS-extended clinical and neuropathological spectrum. Brain Pathol 16:228–234
Roeber S, Mackenzie IR, Kretzschmar HA, Neumann M (2008) TDP-43-negative FTLD-U is a significant new clinico-pathological subtype of FTLD. Acta Neuropathol 116:147–157
Rohrer JD, Lashley T, Holton J et al (2010) The clinical and neuroanatomical phenotype of FUS associated frontotemporal lobar degeneration. J Neurol Neursurg Psychiatry. doi:10.1136/jnnp.2010.214437
Seelar H, Klijnsma KY, de Koning I et al (2010) Frequency of ubiquitin and FUS-positive, TDP-43-negative frontotemporal lobar degeneration. J Neurol 257:747–753
Suzuki N, Aoki M, Warita H et al (2010) FALS with FUS mutation in Japan, with early onset, rapid progress and basophilic inclusions. J Hum Genet 55:252–254
Tateishi T, Hokonohara T, Yamasaki R et al (2010) Multiple system degeneration with basophilic inclusions in Japanese ALS patients with FUS mutation. Acta Neuropathol 119:255–364
Ticozzi N, Silani V, LeClerc AL et al (2009) Analysis of FUS gene mutation in familial amyotrophic lateral sclerosis within an Italian cohort. Neurology 73:1180–1185
Tsuchiya K, Matsunaga T, Aoki M et al (2001) Familial amyotrophic lateral sclerosis with posterior column degeneration and basophilic inclusion bodies: a clinical, genetic and pathological study. Clin Neuropathol 20:53–59
Uchikado H, Shaw G, Wang DS, Dickson DW (2005) Screening for neurofilament inclusion disease using alpha-internexin immunohistochemistry. Neurology 64:1658–1659
Urwin H, Josephs KA, Rohrer JD et al (2010) FUS pathology defines the majority of tau-and TDP-43-negative frontotemporal lobar degeneration. Acta Neuropathol 120:33–41
Vance C, Rogelj B, Hortobagyi T et al (2009) Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323:1208–1211
Van Langenhove T, van der Zee J, Sleegers K et al (2010) Genetic contribution of FUS to frontotemporal lobar degeneration. Neurology 74:366–371
Yamamoto-Watanabe Y, Watanabe M, Okamoto K et al (2010) A Japanese ALS6 family with mutation R521C in the FUS/TLS gene: a clinical, pathological and genetic report. J Neurol Sci 296:59–63
Yan J, Deng HX, Siddique N et al (2010) Frameshift and novel mutations in FUS in familial amyotrophic lateral sclerosis and ALS/dementia. Neurology 75:807–814
Yang S, Warraich ST, Nicholson GA, Blair IP (2010) Fused in sarcoma/translocated in liposarcoma: a multifunctional DNA/RNA binding protein. Int J Biochem Cell Biol 42:1408–1411
Yokota O, Tsuchiya K, Terada S et al (2008) Basophilic inclusion body disease and neuronal intermediate filament inclusion disease: a comparative clinicopathological study. Acta Neuropathol 115:561–575
Zinszner H, Sok J, Immanuel D, Yin Y, Ron D (1997) TLS (FUS) binds RNA in vivo and engages in nucleo-cytoplasmic shuttling. J Cell Sci 110:1741–1750
Acknowledgments
We thank Margaret Luk and Mareike Schroff for their excellent technical assistance. This work was supported by grants from Canadian Institutes of Health Research (grant number 74580, IM); the Pacific Alzheimer Research Foundation (IM); the German Federal Ministry of Education and Research (grant number 01GI0704, MN); the Stavros-Niarchos Foundation (MN); the Synapsis Foundation (MN); and the German Brain Bank “BrainNet” (HK).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mackenzie, I.R.A., Munoz, D.G., Kusaka, H. et al. Distinct pathological subtypes of FTLD-FUS. Acta Neuropathol 121, 207–218 (2011). https://doi.org/10.1007/s00401-010-0764-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-010-0764-0