Abstract
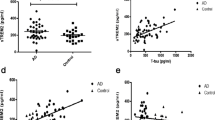
There is mounting pathological, biochemical and genetic evidence that the metabolism and aggregation of the 43-kDa transactive response (TAR)-DNA-binding protein (TDP-43) play a crucial role in the pathogenesis of sporadic and some forms of familial amyotrophic lateral sclerosis (ALS). Recently, it was reported using an ELISA system that elevated levels of TDP-43 were detected in plasma samples from patients with Alzheimer’s disease and frontotemporal dementia, compared to healthy controls. To determine whether quantification of TDP-43 in cerebrospinal fluid (CSF) is potentially informative in the diagnosis of ALS, we measured the concentration, by a similar ELISA method, of TDP-43 in CSF from 30 patients with ALS (diagnosed according to the revised El Escorial criteria) and 29 age-matched control patients without any neurodegenerative disease. We found that, as a group, the ALS patients had significantly higher levels of TDP-43 in their CSF than the age-matched controls (6.92 ± 3.71 ng/ml in ALS versus 5.31 ± 0.94 ng/ml in controls, p < 0.05), with levels of TDP-43 in CSF elevated beyond 95% upper confidence level for the control group in six (20%) of the patients with sporadic ALS. All the six patients with higher levels of CSF TDP-43 were examined within 10 months of the onset of illness. The patients examined within 10 months of onset showed significantly higher levels of CSF TDP-43 (8.24 ± 4.72 ng/ml) than those examined after 11 months or more of onset (5.41 ± 0.66 ng/ml, p < 0.05). These results suggest that the levels of TDP-43 in CSF may increase in the early stage of ALS. We also confirmed the existence of the TDP-43 protein in CSF from some patients with ALS, and a control subject, by western blotting of proteins immunocaptured from the CSF samples. Raised TDP-43 levels in the CSF may preempt the formation of TDP-43 pathology in the central nervous system, or correlate with early-stage TDP-43 pathology, and accordingly be a biomarker for the early stage of ALS.



Similar content being viewed by others
References
Abe K, Fujimura H, Toyooka K, Sakoda S, Yorifuji S, Yanagihara T (1997) Cognitive function in amyotrophic lateral sclerosis. J Neurol Sci 148:95–100. doi:10.1016/S0022-510X(96)05338-5
Arai T, Hasegawa M, Akiyama H et al (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351:602–611. doi:10.1016/j.bbrc.2006.10.093
Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299. doi:10.1080/146608200300079536
Buratti E, Dörk T, Zuccato E, Pagani F, Romano M, Baralle FE (2001) Nuclear factor TDP-43 and SR proteins promote in vitro and in vivo CFTR exon 9 skipping. EMBO J 20:1774–1784. doi:10.1093/emboj/20.7.1774
Cairns NJ, Bigio EH, Mackenzie IR, Consortium for Frontotemporal Lobar Degeneration et al (2007) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 114:5–22. doi:10.1007/s00401-007-0237-2
Cairns NJ, Neumann M, Bigio EH et al (2007) TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol 171:227–240. doi:10.2353/ajpath.2007.070182
Davidson Y, Kelley T, Mackenzie IR et al (2007) Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol 113:521–533. doi:10.1007/s00401-006-0189-y
Dickson DW, Josephs KA, Amador-Ortiz C (2007) TDP-43 in differential diagnosis of motor neuron disorders. Acta Neuropathol 114:71–79. doi:10.1007/s00401-007-0234-5
Foulds P, McAuley E, Gibbons L et al (2008) TDP-43 protein in plasma may index TDP-43 brain pathology in Alzheimer’s disease and frontotemporal lobar degeneration. Acta Neuropathol 116:141–146. doi:10.1007/s00401-008-0389-8
Gitcho MA, Baloh RH, Chakraverty S et al (2008) TDP-43 A315T mutation in familial motor neuron disease. Ann Neurol 63:535–538. doi:10.1002/ana.21344
Gros-Louis F, Gaspar C, Rouleau GA (2006) Genetics of familial and sporadic amyotrophic lateral sclerosis. Biochim Biophys Acta 1762:956–972
Guiloff RJ, Goonetilleke A (1995) Natural history of amyotrophic lateral sclerosis. Observations with the Charing Cross Amyotrophic Lateral Sclerosis Rating Scales. Adv Neurol 68:185–198
Kabashi E, Valdmanis PN, Dion P et al (2008) TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet 40:572–574. doi:10.1038/ng.132
Kwong LK, Neumann M, Sampathu DM, Lee VM, Trojanowski JQ (2007) TDP-43 proteinopathy: the neuropathology underlying major forms of sporadic and familial frontotemporal lobar degeneration and motor neuron disease. Acta Neuropathol 114:63–70. doi:10.1007/s00401-007-0226-5
Lipton AM, White CL 3rd, Bigio EH (2004) Frontotemporal lobar degeneration with motor neuron disease-type inclusions predominates in 76 cases of frontotemporal degeneration. Acta Neuropathol 108:379–385. doi:10.1007/s00401-004-0900-9
Mackenzie IR, Bigio EH, Ince PG et al (2007) Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol 61:427–434. doi:10.1002/ana.21147
Mercado PA, Ayala YM, Romano M, Buratti E, Baralle FE (2005) Depletion of TDP 43 overrides the need for exonic and intronic splicing enhancers in the human apoA-II gene. Nucleic Acids Res 33:6000–6010. doi:10.1093/nar/gki897
Mott RT, Dickson DW, Trojanowski JQ et al (2005) Neuropathologic, biochemical, and molecular characterization of the frontotemporal dementias. J Neuropathol Exp Neurol 64:420–428
Neumann M, Sampathu DM, Kwong LK et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133. doi:10.1126/science.1134108
Nishihira Y, Tan CF, Onodera O et al (2008) Sporadic amyotrophic lateral sclerosis: two pathological patterns shown by analysis of distribution of TDP-43-immunoreactive neuronal and glial cytoplasmic inclusions. Acta Neuropathol 116:169–182. doi:10.1007/s00401-008-0385-z
Nishihira Y, Tan CF, Hoshi Y et al. (2008) Sporadic amyotrophic lateral sclerosis of long duration is associated with relatively mild TDP-43 pathology. Acta Neuropathol. doi:10.1007/s00401-008-0443-6
Otto M, Wiltfang J, Tumani H et al (1997) Elevated levels of tau-protein in cerebrospinal fluid of patients with Creutzfeldt–Jakob disease. Neurosci Lett 225:210–212. doi:10.1016/S0304-3940(97)00215-2
Ou SH, Wu F, Harrich D, García-Martínez LF, Gaynor RB (1995) Cloning and characterization of a novel cellular protein, TDP-43, that binds to human immunodeficiency virus type 1 TAR DNA sequence motifs. J Virol 69:3584–3596
Pamphlett R, Kum Jew S (2008) TDP-43 inclusions do not protect motor neurons from sporadic ALS. Acta Neuropathol 116:221–222. doi:10.1007/s00401-008-0392-0
Pasinelli P, Brown RH (2006) Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci 7:710–723. doi:10.1038/nrn1971
Piao YS, Wakabayashi K, Kakita A et al (2003) Neuropathology with clinical correlations of sporadic amyotrophic lateral sclerosis: 102 autopsy cases examined between 1962 and 2000. Brain Pathol 13:10–22
Ringholz GM, Appel SH, Bradshaw M, Cooke NA, Mosnik DM, Schulz PE (2005) Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology 65:586–590. doi:10.1212/01.wnl.0000172911.39167.b6
Seelaar H, Schelhaas HJ, Azmani A et al (2007) TDP-43 pathology in familial frontotemporal dementia and motor neuron disease without Progranulin mutations. Brain 130:1375–1385. doi:10.1093/brain/awm024
Shaw CE, Al-Chalabi A, Leigh N (2001) Progress in the pathogenesis of amyotrophic lateral sclerosis. Curr Neurol Neurosci Rep 1:69–76. doi:10.1007/s11910-001-0078-7
Shi J, Shaw CL, Du Plessis D et al (2005) Histopathological changes underlying frontotemporal lobar degeneration with clinicopathological correlation. Acta Neuropathol 110:501–512. doi:10.1007/s00401-005-1079-4
Sreedharan J, Blair IP, Tripathi VB et al (2008) TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319:1668–1672. doi:10.1126/science.1154584
Tan CF, Eguchi H, Tagawa A et al (2007) TDP-43 immunoreactivity in neuronal inclusions in familial amyotrophic lateral sclerosis with or without SOD1 gene mutation. Acta Neuropathol 113:535–542. doi:10.1007/s00401-007-0206-9
Vigo-Pelfrey C, Seubert P, Barbour R et al (1995) Elevation of microtubule-associated protein tau in the cerebrospinal fluid of patients with Alzheimer’s disease. Neurology 45:788–793
Yokoseki A, Shiga A, Tan CF et al (2008) TDP-43 mutation in familial amyotrophic lateral sclerosis. Ann Neurol 63:538–542. doi:10.1002/ana.21392
Zhang H, Tan CF, Mori F et al (2008) TDP-43-immunoreactive neuronal and glial inclusions in the neostriatum in amyotrophic lateral sclerosis with and without dementia. Acta Neuropathol 115:115–122. doi:10.1007/s00401-007-0285-7
Acknowledgments
This work was supported by Grant-in-Aid from the Research Committee of CNS Degenerative Disease, the Ministry of Health, Labor, and Welfare of Japan (to M.N.) and by Grant-in-Aid for Scientific Research (C) (20591007), the Ministry of Education, Culture, Sports, Science and Technology and Japan Society for the Promotion of Science (to T.T.). P·F. is supported by a grant from The Medical Research Council, UK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kasai, T., Tokuda, T., Ishigami, N. et al. Increased TDP-43 protein in cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Acta Neuropathol 117, 55–62 (2009). https://doi.org/10.1007/s00401-008-0456-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-008-0456-1