Abstract
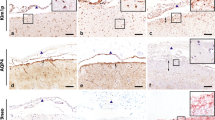
Aquaporin-4 (AQP4) has recently been implicated in the pathogenesis of neuromyelitis optica (NMO) where it has been identified as the first defined autoantigen pertinent to an inflammatory demyelinating disorder of the human CNS. Furthermore, a recent case report has shown a lack of AQP4 expression in the spinal cord lesions of NMO. However, the pattern of AQP4 expression in multiple sclerosis (MS) tissues has not been well-defined. In the present investigation we have confirmed a lack of expression of AQP4 in optic and spinal cord lesions in NMO which contrasted sharply with the increased levels of AQP4 expression seen in MS lesions. Furthermore a detailed immunohistochemical and semi-quantitative analysis is used to describe the expression pattern of AQP4 on well-characterized tissue microarray samples of MS and control white matter. Anatomically AQP4 was more highly expressed in all categories of MS tissue compared to normal control tissues with the most abundant expression in active lesions. Within active lesions AQP4 expression was significantly correlated with expression of the pro-inflammatory cytokine osteopontin. At the cellular level dual-labeling immunofluoresence demonstrated that increased expression of AQP4 was most pronounced at the astrocytic endfeet but was also associated with the cell bodies of astrocytes in the tissue parenchyma. The finding of increased AQP4 expression in MS lesions in contrast to the lack of expression in NMO lesions may suggest different mechanisms of initiation and progression between the two disease states.



Similar content being viewed by others
References
Aboul-Enein F, Rauschka H, Kornek B, Stadelmann C, Stefferl A, Bruck W, Lucchinetti C, Schmidbauer M, Jellinger K, Lassmann H (2003) Preferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J Neuropathol Exp Neurol 62:25–33
Adams CWM (1977) Pathology of multiple sclerosis: progression of the lesion. British Med Bull 33:15–20
Aoki-Yoshino K, Uchihara T, Duyckaerts C, Nakamura A, Hauw JJ, Wakayama Y (2005) Enhanced expression of aquaporin 4 in human brain with inflammatory diseases. Acta Neuropathol (Berl) 110:281–288
Aoki K, Uchihara T, Tsuchiya K, Nakamura A, Ikeda K, Wakayama Y (2003) Enhanced expression of aquaporin 4 in human brain with infarction. Acta Neuropathol (Berl) 106:121–124
Barkhof F, van Walderveen M (1999) Characterization of tissue damage in multiple sclerosis by nuclear magnetic resonance. Philos Trans R Soc Lond B Biol Sci. 354:1675–1686
Christiansen P, Gideon P, Thomsen C, Stubgaard M, Henriksen O, Larsson HB (1993) Increased water self-diffusion in chronic plaques and in apparently normal white matter in patients with multiple sclerosis. Acta Neurol Scand 87:195–199
Davies DC (2002) Blood-brain barrier breakdown in septic encephalopathy and brain tumours. J Anat 200:639–646
Graumann U, Reynolds R, Steck AJ, Schaeren-Wiemers N (2003) Molecular changes in normal appearing white matter in multiple sclerosis are characteristic of neuroprotective mechanisms against hypoxic insult. Brain Pathol 13:554–573
Kobayashi H, Yanagita T, Yokoo H, Wada A (2004) Molecular mechanisms and drug development in aquaporin water channel disease: aquaporins in the brain. J Pharmacol Sci 96:264–270
Lassmann H (2003) Hypoxia-like tissue injury as a component of multiple sclerosis lesions. J Neurol Sci 206:187–191
Lassmann H, Reindl M, Rauschka H, Berger J, Aboul-Enein F, Berger T, Zurbriggen A, Lutterotti A, Bruck W, Weber JR, Ullrich R, Schmidbauer M, Jellinger K, Vandevelde M (2003) A new paraclinical CSF marker for hypoxia-like tissue damage in multiple sclerosis lesions. Brain 126:1347–1357
Laule C, Vavasour IM, Moore GR, Oger J, Li DK, Paty DW, McKay AL (2004) Water content and myelin water fraction in multiple sclerosis. A T2 relaxation study. J Neurol 251:284–293
Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR (2005) IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 202:473–477
Lucchinetti C, Bruck W, Noseworthy J (2001) Multiple sclerosis; recent developments in neuropathology, pathogenesis, magnetic resonance imaging studies and treatment. Curr Opin Neurol 14:259–269
Markovic-Plese S, McFarland HF (2001) Immunopathogenesis of the multiple sclerosis lesion. Curr Neurol Neurosci Rep 1:257–262
Misu T, Fujihara K, Nakamura M, Murakami K, Endo M, Konno H, Itoyama Y (2006) Loss of aquaporin-4 in active perivascular lesions in neuromyelitis optica: a case report. Tohoku J Exp Med 209:269–275
Nicchia GP, Nico B, Camassa LMA, Mola MG, Loh N, Dermietzel R, Spray DC, Svelto M, Frigeri A (2004) The role of aquaporin-4 in blood-brain barrier development and integrity: studies in animal and cell culture models. Neuroscience 129:935–945
Nico B, Frigeri A, Nicchia GP, Corsi P, Ribatti D, Quondamatteo F, Herken R, Girolamo F, Marzullo A, Svelto M, Roncali L (2003) Severe alterations of endothelial and glial cells in the blood-brain barrier of dystrophic mdx mice. Glia 42:235–251
Papadopoulos M (2002) Aquaporin water channels and brain edema. Mount Sinai J Med 69:242–248
Poser CM (1994) Notes on the pathogenesis of multiple sclerosis. Clin Neurosci 2:258–265
Ribeiro MC, Hirt L, Bogousslavsky J, Regli L, Badaut J (2006) Time course of aquaporin expression after transient focal cerebral ischemia in mice. J Neurosci Res 83:1231–1240
Rodriguez A, Perez-Gracia E, Espinosa JC, Pumarola M, Torres JM, Ferrer I (2006) Increased expression of water channel aquaporin 1 and aquaporin 4 in Creutzfeldt-Jakob disease and in bovine spongiform encephalopathy-infected bovine PrP transgenic mice. Acta Neuropathol (Berl) 112:573–585
Saadoun S, Papadopoulos MC, Davies DC, Krishna S, Bell BA (2002) Aquaporin-4 expression is increased in oedematous human brain tumours. J Neurol Neurosurg Psych 72:262–265
Saadoun S, Papadopoulos MC, Krishna S (2003) Water transport becomesuncoupled from K+ siphoning in brain contusion, bacterial meningitis, and brain tumours: immunohistochemical case review. J Clin Pathol 56:972–975
Sinclair C, Mirakhur M, Kirk J, Farrell M, McQuaid S (2005) Up-regulation of osteopontin and alphaBeta-crystallin in the normal-appearing white matter of multiple sclerosis: an immunohistochemical study utilizing tissue microarrays. Neuropathol Appl Neurobiol 31:292–303
Suzuki R, Okuda M, Asai J, Nagashima G, Itokawa H, Matsunaga A, Fujimoto T, Suzuki T (2006) Astrocytes co-express aquaporin-1, -4 and vascular endothelial growth factor in brain edema tissue associated with brain contusion. Acta Neurochir Suppl 96:398–401
Tanaka F, Ozawa Y, Inage Y, Deguchi K, Itoh M, Imai Y, Kohsaka S, Takashima S (2000) Association of osteopontin with ischemic axonal death in periventricular leukomalacia. Acta Neuropathol (Berl) 100:69–74
Taniguchi M, Yamashita T, Kumura E, Tamatani M, Kobayashi A, Yokawa T, Maruno M, Kato A, Ohnishi T, Kohmura E, Tohyama M, Yoshimine T (2000) Induction of aquaporin-4 water channel mRNA after focal ishemia in rat. Brain Res Mol Brain Res 78:131–137
Venero Jl, Vizuete ML, Machado A, Cano J (2001) Aquaporins in the central nervous system. Prog Neurobiol 63:321–336
Wang X, Louden C, Yue TL, Ellison JA, Barone FC, Solleveld HA, Feurerstein GZ (1988) Delayed expression of osteopontin after focal stroke in the rat. J Neurosci 18:2075–2083
Warth A, Mittelbronn M, Wolburg H (2005) Redistribution of the water channel protein aquaporin-4 and the K+ channel protein Kir4.1 differs in low- and high-grade human brain tumours. Acta Neuropathol (Berl) 109:418–426
Acknowledgements
The authors wish to thank MS Ireland for grant funding. We thank Mr. Gordon McGregor for his excellent technical assistance. We also are indebted to Dr Raeburn Forbes and Dr Rory Convery for neurological diagnosis of the NMO case.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sinclair, C., Kirk, J., Herron, B. et al. Absence of aquaporin-4 expression in lesions of neuromyelitis optica but increased expression in multiple sclerosis lesions and normal-appearing white matter. Acta Neuropathol 113, 187–194 (2007). https://doi.org/10.1007/s00401-006-0169-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-006-0169-2