Abstract
Introduction
One of the most important unanswered questions in pediatric hydrocephalus is determining whether treatment with endoscopic third ventriculostomy (ETV) versus shunt results in improved health status and quality of life (QOL). To answer this, the International Infant Hydrocephalus Study (IIHS) was started in 2005 as a prospective, multicenter study to compare ETV and shunt in infants (< 24 months old) with symptomatic triventricular hydrocephalus from aqueductal stenosis. Herein, we present the 5-year primary outcome results.
Methods
IIHS utilized a prospective comprehensive cohort design, in which patients received ETV or shunt, based on either randomization or parental preference. For this analysis, we pooled the randomized arm and the parental preference arm, analyzing them together. At 5 years of age, children were assessed with the Health Utilities Index Mark 2 (HUI-2) (primary outcome) and the Hydrocephalus Outcome Questionnaire (HOQ), a measure of QOL. Results were compared in an analysis of covariance, adjusting for baseline variables including age at surgery and baseline development status.
Results
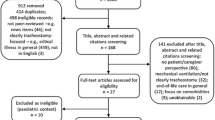
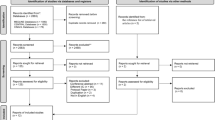
From a total of 158 patients who met eligibility criteria, complete 5-year outcomes were available on 78 (19 treated initially with shunt, 61 treated initially with ETV), assessed at a mean age of 62.1 months (SD 6.3). The mean 5-year HUI-2 utility score was 0.90 (SD 0.19) for ETV and 0.94 (SD 0.10) for shunt (p = 0.21). The mean 5-year HOQ overall score was 0.81 (SD 0.15) for ETV and 0.85 (SD 0.12) for shunt (p = 0.42). Similarly, there were no significant differences noted between 5-year HOQ subscores (cognitive, social-emotional, physical) or developmental measures at 1, 2, and 3 years.
Conclusions
This is the first prospective direct comparison of long-term outcomes of ETV and shunt for infant hydrocephalus. These results suggest that overall health status and quality of life in this cohort of infants treated for aqueductal stenosis are high, with no significant difference between those treated initially with ETV or shunt.
Trial registration
NCT00652470

Similar content being viewed by others
References
Kulkarni AV, Sgouros S, Constantini S (2016) International Infant Hydrocephalus Study: initial results of a prospective, multicenter comparison of endoscopic third ventriculostomy (ETV) and shunt for infant hydrocephalus. Childs Nerv Syst 32:1039–1048. https://doi.org/10.1007/s00381-016-3095-1
Kulkarni AV, Sgouros S, Constantini S, International Infant Hydrocephalus Study Investigators (2017) Outcome of treatment after failed endoscopic third ventriculostomy (ETV) in infants with aqueductal stenosis: results from the International Infant Hydrocephalus Study (IIHS). Childs Nerv Syst 33:747–752. https://doi.org/10.1007/s00381-017-3382-5
Sgouros S, Kulkharni AV, Constantini S (2006) The international infant hydrocephalus study: concept and rational. Childs Nerv Syst 22:338–345. https://doi.org/10.1007/s00381-005-1253-y
O’Hayon BB, Drake JM, Ossip MG, Tuli S, Clarke M (1998) Frontal and occipital horn ratio: a linear estimate of ventricular size for multiple imaging modalities in pediatric hydrocephalus. Pediatr Neurosurg 29:245–249
Kulkarni AV, Drake JM, Armstrong DC, Dirks PB (1999) Measurement of ventricular size: reliability of the frontal and occipital horn ratio compared to subjective assessment. Pediatr Neurosurg 31:65–70
Frankenburg WK, Dodds J, Archer P, Shapiro H, Bresnick B (1992) The Denver II: a major revision and restandardization of the Denver Developmental Screening Test. Pediatrics 89:91–97
Kulkarni AV, Drake JM, Rabin D, Dirks PB, Humphreys RP, Rutka JT (2004) Measuring the health status of children with hydrocephalus by using a new outcome measure. J Neurosurg 101:141–146. https://doi.org/10.3171/ped.2004.101.2.0141
Kulkarni AV, Rabin D, Drake JM (2004) An instrument to measure the health status in children with hydrocephalus: the Hydrocephalus Outcome Questionnaire. J Neurosurg 101:134–140. https://doi.org/10.3171/ped.2004.101.2.0134
Kulkarni AV (2010) Quality of life in childhood hydrocephalus: a review. Childs Nerv Syst 26:737–743. https://doi.org/10.1007/s00381-010-1131-0
Kulkarni AV, Cochrane DD, McNeely PD, Shams I (2008) Medical, social, and economic factors associated with health-related quality of life in Canadian children with hydrocephalus. J Pediatr 153:689–695. https://doi.org/10.1016/j.jpeds.2008.04.068
Kulkarni AV (2006) Distribution-based and anchor-based approaches provided different interpretability estimates for the Hydrocephalus Outcome Questionnaire. J Clin Epidemiol 59:176–184
Feeny D (2000) A utility approach to the assessment of health-related quality of life. Med Care 38(Suppl):151–154
Feeny D, Furlong W, Barr RD, Torrance GW, Rosenbaum P, Weitzman S (1992) A comprehensive multiattribute system for classifying the health status of survivors of childhood cancer. J Clin Oncol 10:923–928
Feeny D, Furlong W, Saigal S, Sun J (2004) Comparing directly measured standard gamble scores to HUI2 and HUI3 utility scores: group- and individual-level comparisons. Soc Sci Med 58:799–809
Kulkarni AV, Shams I, Cochrane DD, McNeely PD (2010) Quality of life after endoscopic third ventriculostomy and cerebrospinal fluid shunting: an adjusted multivariable analysis in a large cohort. J Neurosurg Pediatr 6:11–16. https://doi.org/10.3171/2010.3.PEDS09358
Kulkarni AV, Hui S, Shams I, Donnelly R (2010) Quality of life in obstructive hydrocephalus: endoscopic third ventriculostomy compared to cerebrospinal fluid shunt. Childs Nerv Syst 26:75–79. https://doi.org/10.1007/s00381-009-0983-7
Kulkarni AV, Donnelly R, Mabbott DJ, Widjaja E (2015) Relationship between ventricular size, white matter injury, and neurocognition in children with stable, treated hydrocephalus. J Neurosurg Pediatr 16:267–274. https://doi.org/10.3171/2015.1.PEDS14597
Mandell JG, Kulkarni AV, Warf BC, Schiff Steven J (2014) Volumetric brain analysis in neurosurgery: Part 2. Brain and CSF volumes discriminate neurocognitive outcomes in hydrocephalus. https://doi.org/10.3171/2014.9.PEDS12427
Kulkarni AV, Shams I (2007) Quality of life in children with hydrocephalus: results from the Hospital for Sick Children, Toronto. J Neurosurg 107:358–364. https://doi.org/10.3171/PED-07/11/358
Kulkarni AV, Donnelly R, Shams I (2011) Comparison of Hydrocephalus Outcome Questionnaire scores to neuropsychological test performance in school-aged children. J Neurosurg Pediatr 8:396–401. https://doi.org/10.3171/2011.7.PEDS1179
Platenkamp M, Hanlo PW, Fischer K, Gooskens RH (2007) Outcome in pediatric hydrocephalus: a comparison between previously used outcome measures and the hydrocephalus outcome questionnaire. J Neurosurg 107:26–31. https://doi.org/10.3171/PED-07/07/026
Acknowledgements
The authors would like to extend special thanks to Adina Sherer, who ran the organizational logistics of this study and without whom the IIHS would not have been possible.
Steering committee: Shlomi Constantini (principal investigator), Spyros Sgouros, Abhaya V. Kulkarni
Consultant neurologist: Yael Leitner
Data safety monitoring committee: John RW Kestle (Chair), Douglas D Cochrane, Maurice Choux, Fleming Gjerris
Coordinating administrator: Adina Sherer
Participating investigator authors: Nejat Akalan, Burçak Bilginer (Ankara, Turkey); Ramon Navarro (Barcelona, Spain); Ljiljana Vujotic (Belgrade, Serbia); Hannes Haberl, Ulrich-Wilhelm Thomale (Berlin, Germany); Spyros Sgouros (Birmingham, UK); Graciela Zúccaro, Roberto Jaimovitch (Buenos Aires, Argentina); David Frim, Lori Loftis (Chicago, USA); Dale M. Swift, Brian Robertson, Lynn Gargan (Dallas, USA); László Bognár, László Novák, Georgina Cseke (Debrecen, Hungary); Armando Cama, Giuseppe Marcello Ravegnani (Genova, Italy); Matthias Preuß (Giessen/Leipzig, Germany); Henry W. Schroeder, Michael Fritsch, Joerg Baldauf (Greifswald, Germany); Marek Mandera, Jerzy Luszawski, Patrycja Skorupka (Katowice, Poland); Conor Mallucci, Dawn Williams (Liverpool, UK); Krzysztof Zakrzewski, Emilia Nowoslawska (Lodz, Poland); Chhitij Srivastava, Ashok K. Mahapatra, Raj Kumar, Rabi Narayan Sahu (Lucknow, India); Armen G. Melikian, Anton Korshunov, Anna Galstyan (Moscow, Russia); Ashish Suri, Deepak Gupta (New Delhi, India); J. André Grotenhuis, Erik J. van Lindert (Nijmegen, The Netherlands); José Aloysio da Costa Val (Nova Lima, Brazil); Concezio Di Rocco, Gianpiero Tamburrini (Rome, Italy); Samuel Tau Zymberg, Sergio Cavalheiro (São Paulo, Brazil); Ma Jie, Jiang Feng (Shanghai, China); Shlomi Constantini, Orna Friedman (Tel Aviv, Israel); Abhaya V. Kulkarni, Naheeda Rajmohamed (Toronto, Canada); Marcin Roszkowski, Slawomir Barszcz (Warsaw, Poland)
The following centers (and investigators) participated in the IIHS, but did not enroll any patients: Baltimore, MD, USA (George Jallo); Gainesville, FL, USA (David W. Pincus, Bridget Richter); Kiel, Germany (HM Mehdorn, Susan Schultka); London, Ontario, Canada (Sandrine de Ribaupierre); London, UK (Dominic Thompson, Silvia Gatscher); Mainz, Germany (Wolfgang Wagner, Dorothee Koch); Reggio Calabria, Italy (Saverio Cipri, Claudio Zaccone); Winnipeg, Manitoba, Canada (Patrick McDonald)
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
The IIHS was publically registered (clinicaltrials.gov, NCT00652470) and received ethics approval from all participating institutions.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Kulkarni, A.V., Sgouros, S., Leitner, Y. et al. International Infant Hydrocephalus Study (IIHS): 5-year health outcome results of a prospective, multicenter comparison of endoscopic third ventriculostomy (ETV) and shunt for infant hydrocephalus. Childs Nerv Syst 34, 2391–2397 (2018). https://doi.org/10.1007/s00381-018-3896-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-018-3896-5