Abstract
Purpose of Review
Pediatric sepsis remains an important cause of morbidity and mortality in children. This review will summarize the main aspects of the definition, the current evidence base for interventions discuss some controversial themes and point towards possible areas of improvement.
Recent Findings
Controversy remains regarding the accurate definition, resuscitation fluid volume and type, choice of vasoactive/inotropic agents, and antibiotic depending upon specific infection risks. Many adjunctive therapies have been suggested with theoretical benefits, although definitive recommendations are not yet supported by data. We describe best practice recommendations based on international guidelines, a review of primary literature, and a discussion of ongoing clinical trials and the nuances of therapeutic choices.
Summary
Early diagnosis and timely intervention with antibiotics, fluid resuscitation, and vasoactive medications are the most important interventions in sepsis. The implementation of protocols, resource-adjusted sepsis bundles, and advanced technologies will have an impact on reducing sepsis mortality.
Similar content being viewed by others
Introduction
Sepsis is a clinical syndrome caused by a dysregulated host response to severe infection. Pediatric sepsis remains a major public health problem and an important cause of morbidity and mortality, despite the development of standardized treatment guidelines, universal immunization programs, and advanced intensive care organ support techniques. Severe sepsis is responsible for > 8% of all pediatric intensive care unit (PICU) admissions and causes > 4.5 million childhood deaths worldwide per year [1••, 2, 3].
Although inflammation is an essential host response to any infection, the progression to dysregulation of the normal host response causes the activation of a chain of events that leads to widespread tissue injury, immune and microcirculatory dysregulation, and characteristics of systemic inflammatory response syndrome (SIRS). This uncontrolled, dysregulated, and self-sustaining intravascular inflammation can lead to end-organ dysfunction in tissues remote from the original insult, progressing rapidly to septic shock with associated multiple organ failure [1••, 4]. If not treated in a timely manner, death will occur either as refractory shock, responsible for one-third of deaths within the first 72 h, or as multiple organ dysfunction syndrome (MODS), with respiratory failure and neurological failure that predominate as the main causes of death [5].
The recognition of sepsis in children is challenging and is related to the high prevalence of common febrile infections, poor specificity of discriminating features, and some capacity of children’s physiology to compensate until shock is in an advanced stage [1••]. Sepsis outcomes in children are strongly dependent on the timeliness of recognition and treatment. Several worldwide campaigns and recommendations emphasize early recognition and timely diagnosis of sepsis, collectively with appropriate and timely management consisting of prompt use of empiric antimicrobials and early escalation of care [1••, 6]. Although this has been shown to reduce mortality, mortality rates remain practically unchanged in high-income settings [2].
Although most pediatric sepsis studies are either small, retrospective, or observational, some important new evidence has been produced in the last few years in multiple areas of this subject. This review will briefly summarize some of the current evidence-based interventions for pediatric sepsis, discuss controversial aspects, and point towards possible areas of improvement.
Evolving Definitions of Sepsis
The definition of pediatric sepsis is still an immense challenge and without consensus. The last published definitions for pediatric sepsis, severe sepsis, and septic shock in children are based on the 2005 International Pediatric Sepsis Consensus Conference (Table 1) [7]. Pediatric definitions remain despite the new 2016 adult definitions and criteria (Sepsis-3), where “sepsis” is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection and “septic shock” is a subset of sepsis with circulatory and cellular/metabolic dysfunction associated with a higher risk of mortality [8]. There is ongoing debate regarding whether these adult definitions are applicable to children [9].
The most recent meta-analysis reviewing the criteria for pediatric sepsis was published in 2021 by the Pediatric Sepsis Definition Taskforce. It revealed strong associations of several markers of organ dysfunction with outcomes, including: in children with infection, decreased level of consciousness and higher Pediatric Risk of Mortality scores are associated with sepsis/severe sepsis; in children with sepsis/severe sepsis/septic shock, chronic conditions, oncologic diagnosis, use of vasoactive/inotropic agents, mechanical ventilation, serum lactate, platelet count, fibrinogen, procalcitonin, multi-organ dysfunction syndrome, Pediatric Logistic Organ Dysfunction score, Pediatric Index of Mortality-3, and Pediatric Risk of Mortality score each demonstrated significant and consistent associations with mortality [10••].
For the purposes of this article, septic shock in children is defined as severe infection leading to cardiovascular dysfunction (including hypotension, need for treatment with a vasoactive medication, or impaired perfusion), and “sepsis-associated organ dysfunction” in children is defined as severe infection leading to cardiovascular and/or non-cardiovascular organ dysfunction, as the majority of studies used to establish evidence refer to this nomenclature.
Screening, Diagnosis, and Initial Management of Sepsis
Pediatric sepsis is associated with high mortality and morbidity and requires a high level of awareness and suspicion for early diagnosis and timely treatment. Rapid and careful fluid resuscitation, antibiotic administration, and early vasoactive support are critical to reversing shock. The Surviving Sepsis Campaign published very comprehensive guidelines in 2020 for the management of pediatric septic shock and sepsis-associated organ dysfunction (summary in Table 2) that include recommendations for the screening and diagnosis of sepsis [1••].
Sepsis causes hypovolemia due to capillary leak, vasodilation and fluid loss to the third space. Evidence of inadequate oxygen delivery and tissue perfusion (skin, brain, and kidneys) often accompanies sepsis in children. However, early identification of sepsis in children can be very difficult as the early symptoms may be very non-specific. Tachycardia is a sensitive, though non-specific, indicator often seen in early stages of shock. In the other hand, hypotension can be a late sign of shock in infants and children (and its presence is not necessary for the diagnosis), who often maintain cardiac output despite the presence of shock through an increase in heart rate, systemic vascular resistance, and venous tone, but have a limited capacity to augment myocardial stroke volume [1••, 11]. Barriers to recognition include age-related variation in vital signs, a relatively low prevalence of pediatric sepsis in high-income countries, and alternative more common explanations for abnormal vital signs (fever or crying contributing to tachycardia or tachypnea) [12]. Laboratory markers can be helpful, including blood lactate, full blood count, CRP, procalcitonin, platelet count, clotting screen, renal and liver function tests, and blood culture [12, 13]. Serum lactate > 2 mmol/L (> 18 mg/dL) suggests hypoperfusion and is a component of the adult Sepsis-3 definition of septic shock. Studies have reported that increasing lactate levels are associated with a higher risk of MODS and mortality in children with infection, in particular, if > 4 mmol/L (> 36 mg/dL). Though, normal lactate does not exclude a sepsis diagnosis in children [14, 15].
A large number of health care systems now use Pediatric Early Warning Scores (PEWS) in both ED as well as on the ward, which help to improve early identification of the deteriorating child [12, 16]. There are also associated challenges in identifying which children meeting SIRS criteria may be at risk for sepsis, and so it is essential to review a thorough history to ascertain whether the patient has risk factors for sepsis.
The initial management of septic shock is as for all other life-threatening conditions, with airway stabilization and adequate breathing with extra oxygen supply to maintain appropriate tissue oxygen delivery. It can be difficult to quickly identify which patients have severe circulatory volume compromise. It is important to establish vascular access as soon as possible, provide early cautious fluid replacement trials, followed by early vasoactive agents to improve cardiac contractility, and ultimately improve perfusion [1••, 11].
Sepsis Bundles and Quality Improvement Initiatives
Institutional implementation of evidence-based resuscitation protocols, screening tools, and sepsis “bundles of care” with transparent goals have been shown to improve early identification of the septic child, adherence to best practices, decrease time to therapy, and improve outcomes in pediatric septic shock. These usually consist of protocol-driven care to assist in sepsis recognition and subsequently prompt initiation of treatment. These are associated with improved outcomes that extend beyond reductions in mortality. Various studies have demonstrated decreased hospital length of stay and reduction of acute kidney injury [1••, 5, 17,18,19]. Children who experienced antibiotic delays of more than 3 h presented almost a fourfold risk of mortality in the PICU [20]. Other studies have shown that each additional hour of persistent shock is associated with a > twofold increased odds of mortality [21]. Nevertheless, these bundles have been assessed mainly in high-income settings and need further validation in other settings.
Ongoing Organ Support in the First Hour
Early consideration of escalation and admission to PICU is essential. In children who have no evidence of cardiovascular compromise, fluid bolus therapy should not be given and maintenance fluid therapy should be started instead. In children with shock, fluid boluses of 10–20 mL/kg aliquots should be administered, with close monitoring of heart rate, capillary refill time, blood pressure, urine output, and blood lactate level. The child should be re-assessed regularly following each fluid bolus to evaluate response and check for signs of fluid overload, including new or worsening hepatomegaly, new or increasing oxygen requirement, basal crepitations, or radiographic evidence of pulmonary edema. Fluid boluses up to 60 mL/kg can be given within the first hour in settings with access to intensive care [1••, 11, 22]. The FEAST study in East Africa demonstrated that in lower resource settings that cannot provide an advanced airway and circulatory support, fluid bolus therapy should be given with greater caution, reserved for patients with hypotension, and should not exceed 40 mL/kg in the first hour [1••, 23].
While there is limited data in the pediatric population, randomized control trials in adults have shown that the use of crystalloid fluids containing high concentrations of chloride for resuscitation is associated with an increased risk of hyperchloremic acidosis, acute kidney injury, coagulopathy, and mortality when compared with balanced or buffered crystalloid solutions such as Ringer’s lactate or PlasmaLyte [1••, 24,25,26]. The routine use of colloid solutions such as 5% human albumin solution or gelofusin is not recommended, as they have not shown advantages over crystalloids, are more expensive, less easily available, and carry an increased risk of infection or coagulopathy [1••, 27].
It is important to anticipate the need for concomitant administration of vasoactive drugs in fluid-refractory shock (children who have received 40–60 mL/kg of fluid resuscitation within an hour and who remain shocked). In these children, the initiation of vasoactive medications should not be delayed and should be instituted concomitantly and independently of volume resuscitation [1••, 28].
Studies have shown increased adverse effects with the use of dopamine in shock compared to epinephrine and norepinephrine [28,29,30]. As there are few studies of poor quality that compare the use of epinephrine and norepinephrine in children with fluid refractory shock, the choice of agent depends on the treating clinician’s preference, local policy, and an assessment of physiology. Epinephrine (initial starting dose 0.05 to 0.1 mcg/kg/min) is often used to manage shock associated with myocardial dysfunction and low cardiac output state. On the other hand, norepinephrine (initial dose, starting dose 0.05 to 0.1 mcg/kg/min) is often used to manage shock where vasodilatation and decreased systemic vascular resistance are present [1••]. Recent studies have shown that in children and adolescents with pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 infection (PIMS-TS/MIS-C), the vasoplegic shock represents a dominant hemodynamic profile, which should be taken into consideration when choosing the vasoactive support need for these patients [31•].
The previously used classification of pediatric septic shock into “warm” (indicating high cardiac output and low systemic vascular resistance) or “cold” (indicating low cardiac output and high systemic vascular resistance) is now outdated as it has been shown that there is poor correlation between clinical assessment, cardiac index, and systemic vascular resistance when measured using advanced monitoring techniques [1••, 32•].
Administration of epinephrine/norepinephrine should be started through peripheral or intraosseous access in dilute concentrations and should not be delayed while awaiting placement of central venous access. Recent studies of the use of peripheral epinephrine/norepinephrine in children with PIMS-TS/MIS-C showed that they are safe to use, even during transport, as long as the access site is closely monitored [33]. Observational studies in hospitalized children receiving administration of vasoactive drugs through a peripheral vein indicate that extravasation occurs in approximately 2% of patients. Suggested dilutions varied, including 0.3 mg/kg in 50 mL diluent for children until 13 kg/4 mg in 50 mL for children over 13 kg for both or 0.8 mg in 50 mL diluent for all ages for norepinephrine [33, 34].
Risk factors for adverse events in children receiving vasoactive therapy through peripheral access include: young age (< 1 year old); small gauge IV (e.g., 24 gauge); hand IV site; increased severity of illness; longer duration (> 3–6 h) of peripheral infusion; higher vasoactive medication doses (e.g., > 10 mcg/kg/min for dopamine or > 0.1 mcg/kg/min epinephrine/norepinephrine) [33]. If patients without risk factors for peripheral vasoactive complications exhibit low illness severity and are anticipated to wean off vasoactive medications within 6 h, placement of a central venous catheter may be avoided [33, 34].
Although there is no definitive data, evidence suggests that vasoactive agents should be titrated in a goal-oriented approach to a mean arterial pressure (MAP) between the 5th and 50th percentile for the age, adequate urine output, and adequate peripheral perfusion [1••].
Vasopressin-receptor agonists (e.g., vasopressin or terlipressin) may be used in catecholamine-resistant shock, and inodilators (e.g., milrinone) can be considered if the child remains in shock with evidence of low cardiac output [35,36,37]. These therapies are typically initiated in the intensive care unit setting, where advanced hemodynamic monitoring is available.
If signs of respiratory distress develop, a trial of noninvasive positive pressure ventilation can be considered for children who lack clear indications for intubation. The decision for intubation and mechanical ventilation should not be delayed in the presence of respiratory failure, altered state of consciousness, or shock refractory to initial management [1••, 38]. Administration of general anesthetic drugs and muscle relaxants, along with the transition to positive pressure ventilation, can reduce venous return and precipitate cardiac arrest.
Anesthetic induction agents that may cause cardiac depression or vasodilatation, such as propofol or benzodiazepines, should be avoided. Etomidate should be avoided also, as small studies have found significant adrenal suppression in adults with sepsis [39]. There are limited data on optimal induction agents. Most authorities recommend the use of ketamine and/or fentanyl as induction agents (the latter administered at lower doses in children with hypotension).
Antimicrobial Therapy
Prompt identification and treatment of the source of infection are the primary therapeutic interventions for septic shock, with most other interventions being purely supportive. Sepsis can be caused by bacterial, viral (these first two being the most common causes), fungal, parasitic, and rickettsial infections. Empiric broad-spectrum parenteral antibiotic therapy should be initiated ideally within an hour of the recognition of septic shock, as evidence suggests improvement in survival. In children with sepsis without shock, the 2020 SSC recommends starting antimicrobial therapy after appropriate evaluation and within 3 h of recognition (Table 3) [1••, 20].
General principles for empiric antimicrobial coverage include: [1••, 20, 40,41,42].
-
Maximize antimicrobial dose by using dosing recommended for severe infection;
-
Multidrug therapy is recommended in immunocompromised patients or immunocompetent patients at high risk for multidrug-resistant pathogens;
-
Children with septic shock at risk for methicillin-resistant Staphylococcus aureus (MRSA) should receive empiric vancomycin or an alternative agent;
-
Coverage for enteric organisms should be added whenever clinical features suggest genitourinary and/or gastrointestinal sources (e.g., perforated appendicitis or bacterial overgrowth in a child with short gut syndrome);
-
Treatment for Pseudomonas species should be included if immunosuppressed;
-
Listeria monocytogenes and herpes simplex virus are important pathogens in infants ≤ 28 days of age;
-
The collection of relevant microbiological specimens (including blood, urine, sputum, and CSF) should not delay antibiotic administration;
-
Ongoing antimicrobial therapy should be modified based on culture results, including antimicrobial susceptibility and the patient's clinical course;
-
Antimicrobial stewardship should be actively employed.
The choice of antimicrobial should be based on known epidemiology and local antimicrobial resistance patterns, travel history and the likely source of infection, presence of any indwelling devices, comorbidities, recent hospital admissions, and known colonization with specific pathogens [40, 42].
As soon as clinically feasible, interventions to achieve source control should be implemented. This may include removal of suspected infected indwelling devices, abscess drainage, debridement of necrotic soft tissue, and drainage of a septic joint or empyema [1••, 41].
Approximately 30–75% of children with sepsis have no infectious etiology identified [42]. This culture-negative sepsis may indicate host response to bacterial components, as endotoxin, or result from antibiotic treatment prior to obtaining bacterial cultures. However, current diagnostic tests may not be sufficiently sensitive to detect the pathogen, and newer molecular diagnostic techniques, as multiplex polymerase chain reaction (PCR), have the potential to improve the rate of organism identification [43].
Antimicrobial stewardship is an important tool for de-escalation of antibiotics to a narrower spectrum as soon as possible, based on clinical improvement, site of infection, and whether source control has been achieved, together with microbiological data, when appropriate to reduce potential drug toxicity and avoidance of prolonged use. It also involves cessation of antimicrobials if an alternative noninfectious etiology is confirmed [1••, 40].
Catecholamine-Resistant Shock
This term refers to patients with fluid-refractory shock who remain shocked despite the use of catecholamines. The authors recommend titrating the catecholamine infusions:
-
Epinephrine infusion should be titrated to respond. Doses above 1 mcg/kg/min suggest non-response. At doses exceeding 0.1 mcg/kg/minute, alpha-adrenergic effects become more prominent, and systemic vasoconstriction may be more evident.
-
Norepinephrine infusion should be titrated to respond. Doses above 1 mcg/kg/min suggest non-response. It acts on alpha-1 and beta-1 adrenergic receptors; hence, it is a potent vasoconstrictor as well as causing a modest increase in cardiac output (although norepinephrine should is not the first choice agent for myocardial dysfunction).
-
If on high dose of epinephrine and norepinephrine, it is also reasonable to consider adding Vasopressin (starting at 0.0005 U/kg/min and titrated to 0.002 U/kg/min). Nevertheless, as vasopressin has been associated with an increased risk of ischemic events without a clear survival benefit, further titrating catecholamines is a reasonable alternative [1••, 35].
-
Consider adding an inodilator (e.g., milrinone) if the child remains in shock with evidence of low cardiac output in the intensive care unit setting where advanced hemodynamic monitoring is available [1••, 36].
-
If the patient is not responding as expected, it is essential to review the infusions and maintain continuous monitoring of peripheral vascular/ intraosseous access for any signs of extravasation.
Children with refractory shock are a population at very high risk for mortality and need urgent evaluation for unrecognized morbidities, including pneumothorax, pericardial effusion, intra-abdominal hypertension, ongoing blood loss, presence of infected source, and overt adrenal insufficiency [37]. Bedside ultrasound of the lungs, heart, and abdomen by a properly trained provider can provide data about fluid responsiveness (estimated by measuring inferior vena cava distensibility), cardiac output (estimated through left ventricular function), and the exclusion of pneumothorax and pericardial effusion [44].
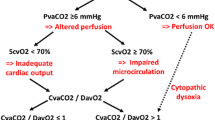
It is essential to provide care in a PICU under continuous monitoring electrocardiographic tracing, pulse oximeter, heart rate, invasive blood pressure, temperature, and urine output. Regular blood gas with monitoring of lactate and electrolytes is required [1••]. Central vascular and arterial access should be obtained as soon as possible. Arterial and superior vena cava oxygen saturation can help guide therapies to maintain mixed venous oxygen saturation (SvO2) above 70% and restore normal perfusion. Cardiac output monitoring to measure cardiac index and systemic vascular resistance may also help guide therapy [45].
Electrolytes should be monitored regularly and optimized when necessary. The routine use of insulin to maintain glucose within a tight range is not recommended. Adjunctive insulin treatment should only be considered if hyperglycemia is associated with clinical compromise despite control of glucose administration [46]. Calcium plays an important role in myocardial contractility; thus, ionized blood calcium levels should be maintained above 1 mmol/L. Hypomagnesemia may exacerbate cardiac dysrhythmias but should be treated cautiously, as magnesium sulfate can worsen hypotension.
Patients at risk for absolute adrenal insufficiency due to purpura fulminans, recent or chronic treatment with corticosteroids, hypothalamic or pituitary abnormalities, or other causes of congenital or acquired adrenal insufficiency should be treated with stress-dose hydrocortisone early in the course of resuscitation (IV hydrocortisone 50 to 100 mg/m2/day or approximately 2 to 4 mg/kg/day). Low-dose hydrocortisone is also commonly used in previously healthy children who remain in refractory shock; however, there is no good evidence of benefit. Such patients may have “critical illness-related corticosteroid insufficiency” [47, 48]. Signs that point to adrenal insufficiency during septic shock include hypoglycemia, hyponatremia, and hyperkalemia. If possible, collect baseline serum cortisol levels before starting hydrocortisone.
In hemodynamically unstable children (e.g., hypotension, persistence of lactate > 2 mmol/L, progressive/persistent end-organ dysfunction, and/or ScvO2 < 70% despite high levels of vasopressor support or profound hypoxia), we suggest blood transfusion to maintain a hemoglobin threshold of 9 g/dL, although evidence for this is poor. For hemodynamically stable children who are not bleeding, the recommendations are to keep a threshold of minimum of 7 g/dL [1••].
Disseminated intravascular coagulopathy (DIC) is common in children with septic shock and may require transfusion with platelets, fresh frozen plasma, and/or cryoprecipitate if actively bleeding. There is no evidence to recommend prophylactic transfusions, even with coagulopathy. The most recent 2022 recommendations and expert consensus for plasma and platelet transfusion practice in critically ill children with sepsis and/or DIC from the Transfusion and Anemia EXpertise Initiative—Control/Avoidance of Bleeding (TAXI-CAB) are the following: [1••, 49••].
-
1.
Do not use prophylactic plasma transfusion in the absence of moderate or severe bleeding;
-
2.
If moderate bleeding, do not use plasma transfusion if the INR is ≤ 1.5;
-
3.
In the absence of moderate or severe bleeding, consider platelet transfusion if platelet count is < 10 × 109/L (10,000/mm3);
-
4.
If moderate bleeding is present, consider platelet transfusion if platelet count is < 50 × 109/L (50,000/mm3).
Advanced Therapies for Refractory Septic Shock
Fluid overload is associated with increased morbidity and likely mortality in critically ill children; however, there is no evidence that routine use of renal replacement therapy (RRT) is associated with improved outcomes. Common indications for initiation of RRT in children with septic shock include fluid overload unresponsive to fluid restriction and diuretic therapy, acute kidney injury, and persistent lactic acidosis [1••, 50, 51]. These patients are at risk of pulmonary edema and development of sepsis-induced pediatric acute respiratory distress syndrome (PARDS). They may require higher (> 10 cm H2O) positive end-expiratory pressure to prevent alveolar collapse and optimize oxygenation, and best practices for PARDS, including prone positioning and consideration for ECMO, should be followed [52, 53]. Inhaled nitric oxide therapy is not routinely recommended, but it should be considered in children with pulmonary hypertension or severe right ventricular dysfunction with refractory hypoxemia despite optimization of oxygenation strategies [1••, 54].
Extracorporeal membrane oxygenation (ECMO) may be used as rescue when conventional respiratory and/or cardiac support prove insufficient. The core concept of ECMO is to deliver enough oxygen to the tissues as determined by continuous recovery of lactate and organ function. Survival rates in patients submitted to ECMO for circulatory instability in septic shock may reach > 50%. Besides improved survival, ECMO has also been shown not to increase severe disability compared with conventional respiratory care. The most common causes of death on ECMO are intracranial bleeding and ischemic events. Treatment of these patients should be concentrated in high-volume ECMO centers experienced in sepsis [1••, 55•, 56].
Future Directions
While there have been many outstanding advancements in pediatric sepsis care, there is much work that remains. This work falls into three broad categories:
-
(1)
advancing QI initiatives beyond dedicated children’s hospitals;
-
(2)
understanding sepsis phenotypes and biomarker profiles and potentially incorporating these into diagnostic and treatment algorithms;
-
(3)
understanding and addressing health disparities.
Implementation of pediatric sepsis guidelines has focused on tertiary care children’s hospitals; however, over 70% of children seeking emergency care are first seen in a general ED, many of which are under-prepared to care for children.
Several groups are exploring sepsis phenotypes, how best to identify and categorize them, and if there are treatment strategies that can be tailored to different phenotypes. There are multiple pediatric and adult-focused randomized clinical trials ongoing to evaluate these questions, paving the way for the entry of personalized medicine into sepsis care. This is a truly exciting area of research that has potential to significantly improve mortality and long-term complications from pediatric sepsis.
Prevention by vaccination has led to major reductions in community-acquired bacterial sepsis in children, but further measures are required, especially efforts to control the spread of antimicrobial-resistant pathogens, if we are to further reduce sepsis-related mortality worldwide.
Conclusion
Even though there has been great progress in the recognition and treatment of sepsis and septic shock in recent years, with implementation of bundle protocols, international guidelines and advanced technologies, sepsis remains a condition with high morbidity and mortality worldwide. A more accurate definition is required for the pediatric population, to help with correct and timely diagnosis, definition of disease stages and identification of specific therapies for each disease evolution stage, as well as to define relevant populations for clinical trials. Until this is available, implementation of evidence-based and resource adjusted sepsis bundles, protocols and guidelines should be encouraged, as these ensure standardized care and improve outcome. The future of pediatric sepsis research should focus on prospective randomized trials that evaluate both in-hospital outcomes as well as long-term outcomes.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Weiss SL, Peters MJ, Alhazzani W, Agus MSD, Flori HR, Inwald DP, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med. 2020;21(2):e52–106. https://doi.org/10.1097/PCC.0000000000002198. The most recent and complete society recommendations.
Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. 2018;6(3):223–30. https://doi.org/10.1016/S2213-2600(18)30063-8.
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–11. https://doi.org/10.1016/S0140-6736(19)32989-7.
Cinel I, Dellinger RP. Advances in pathogenesis and management of sepsis. Curr Opin Infect Dis. 2007;20(4):345–52. https://doi.org/10.1097/QCO.0b013e32818be70a.
Workman JK, Ames SG, Reeder RW, Korgenski EK, Masotti SM, Bratton SL, et al. Treatment of pediatric septic shock with the surviving sepsis campaign guidelines and PICU patient outcomes. Pediatr Crit Care Med. 2016;17(10):e451–8. https://doi.org/10.1097/PCC.0000000000000906.
Tan B, Wong JJ, Sultana R, Koh JC, Jit M, Mok YH, et al. Global case-fatality rates in pediatric severe sepsis and septic shock: a systematic review and meta-analysis. JAMA Pediatr. 2019;173(4):352–62. https://doi.org/10.1001/jamapediatrics.2018.4839.
Goldstein B, Giroir B, Randolph A. International consensus conference on pediatric sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. https://doi.org/10.1097/01.PCC.0000149131.72248.E6.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10. https://doi.org/10.1001/jama.2016.0287.
Morin L, Hall M, de Souza D, Guoping L, Jabornisky R, Shime N, Ranjit S, Gilholm P, Nakagawa S, Zimmerman JJ, Sorce LR, Argent A, Kissoon N, Tissières P, Watson RS, Schlapbach LJ. Pediatric sepsis definition taskforce. The current and future state of pediatric sepsis definitions: an international survey. Pediatrics. 2022;149(6):e2021052565. https://doi.org/10.1542/peds.2021-052565.
•• Menon K, Schlapbach LJ, Akech S, Argent A, Chioto K, Jobayer M, et al. Pediatric sepsis definition-a systematic review protocol by the pediatric sepsis definition taskforce. Crit Care Explor. 2020;2(6):e0123. https://doi.org/10.1097/CCE.0000000000000123. An interesting review of the definition of sepsis.
Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ. 2016;353:i1585. https://doi.org/10.1136/bmj.i1585.
Lambert V, Matthews A, MacDonell R, Fitzsimons J. Paediatric early warning systems for detecting and responding to clinical deterioration in children: a systematic review. BMJ Open. 2017;7(3):e014497. https://doi.org/10.1136/bmjopen-2016-014497. Published 2017 Mar 13.
Downes KJ, Fitzgerald JC, Weiss SL. Utility of procalcitonin as a biomarker for sepsis in children. J Clin Microbiol. 2020;58(7):e01851-e1919. https://doi.org/10.1128/JCM.01851-19.
Scott HF, Brou L, Deakyne SJ, Fairclough DL, Kempe A, Bajaj L. Lactate clearance and normalization and prolonged organ dysfunction in pediatric sepsis. J Pediatr. 2016;170:149-55.e554. https://doi.org/10.1016/j.jpeds.2015.11.071.
Choudhary R, Sitaraman S, Choudhary A. Lactate clearance as the predictor of outcome in pediatric septic shock. J Emerg Trauma Shock. 2017;10(2):55–9. https://doi.org/10.4103/JETS.JETS_103_16.
Parshuram CS, Duncan HP, Joffe AR, et al. Multicentre validation of the bedside paediatric early warning system score: a severity of illness score to detect evolving critical illness in hospitalised children. Crit Care. 2011;15(4):R184. https://doi.org/10.1186/cc10337.
Lane RD, Funai T, Reeder R, Larsen GY. High reliability pediatric septic shock quality improvement initiative and decreasing mortality. Pediatrics. 2016;138(4):e20154153. https://doi.org/10.1542/peds.2015-4153.
Akcan Arikan A, Williams EA, Graf JM, Kennedy CE, Patel B, Cruz AT. Resuscitation bundle in pediatric shock decreases acute kidney injury and improves outcomes. J Pediatr. 2015;167(6):1301-5.e1. https://doi.org/10.1016/j.jpeds.2015.08.044.
Cruz AT, Perry AM, Williams EA, Graf JM, Wuestner ER, Patel B. Implementation of goal-directed therapy for children with suspected sepsis in the emergency department. Pediatrics. 2011;127(3):e758–66. https://doi.org/10.1542/peds.2010-2895.
Weiss SL, Fitzgerald JC, Balamuth F, Alpern ER, Lavelle J, Chilutti M, et al. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med. 2014;42(11):2409–17. https://doi.org/10.1097/CCM.0000000000000509.
Han YY, Carcillo JA, Dragotta MA, Bills DM, Watson RS, Westerman ME, et al. Early reversal of pediatric-neonatal septic shock by community physicians is associated with improved outcome. Pediatrics. 2003;112(4):793–9. https://doi.org/10.1542/peds.112.4.793.
Kleinman ME, de Caen AR, Chameides L, Atkins DL, Berg RA, Berg MD, et al. Pediatric basic and advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Pediatrics. 2010;126(5):e1261–318. https://doi.org/10.1542/peds.2010-2972A.
Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, et al. FEAST trial group. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364(26):2483–95. https://doi.org/10.1056/NEJMoa1101549
Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, et al. Balanced crystalloids versus saline in critically Ill adults. N Engl J Med. 2018;378(9):829–39. https://doi.org/10.1056/NEJMoa1711584.
Emrath ET, Fortenberry JD, Travers C, McCracken CE, Hebbar KB. Resuscitation with balanced fluids is associated with improved survival in pediatric severe sepsis. Crit Care Med. 2017;45(7):1177–83. https://doi.org/10.1097/CCM.0000000000002365.
Weiss SL, Keele L, Balamuth F, Vendetti N, Ross R, Fitzgerald JC, et al. Crystalloid fluid choice and clinical outcomes in pediatric sepsis: a matched retrospective cohort study. J Pediatr. 2017;182:304-310.e10. https://doi.org/10.1016/j.jpeds.2016.11.075.
Upadhyay M, Singhi S, Murlidharan J, Kaur N, Majumdar S. Randomized evaluation of fluid resuscitation with crystalloid (saline) and colloid (polymer from degraded gelatin in saline) in pediatric septic shock. Indian Pediatr. 2005;42(3):223–31.
Ventura AM, Shieh HH, Bousso A, Góes PF, de Cássia FO, Fernandes I, de Souza DC, et al. Double-blind prospective randomized controlled trial of dopamine versus epinephrine as first-line vasoactive drugs in pediatric septic shock. Crit Care Med. 2015;43(11):2292–302. https://doi.org/10.1097/CCM.0000000000001260.
Ramaswamy KN, Singhi S, Jayashree M, Bansal A, Nallasamy K. Double-blind randomized clinical trial comparing dopamine and epinephrine in pediatric fluid-refractory hypotensive septic shock. Pediatr Crit Care Med. 2016;17(11):e502–12. https://doi.org/10.1097/PCC.0000000000000954.
Lampin ME, Rousseaux J, Botte A, Sadik A, Cremer R, Leclerc F. Noradrenaline use for septic shock in children: doses, routes of administration and complications. Acta Paediatr. 2012;101(9):e426–30. https://doi.org/10.1111/j.1651-2227.2012.02725.x.
• Alali A, O’Neil E, Anders M, Abella J, Shekerdemian LS, Vogel TP, et al. Vasoplegic shock represents a dominant hemodynamic profile of multisystem inflammatory syndrome following COVID-19 in children and adolescents. Pediatr Crit Care Med. 2022;23(6):e295–9. https://doi.org/10.1097/PCC.0000000000002954. An elegant paper on this new septic entity.
• Walker SB, Conlon TW, Zhang B, Mensinger JL, Fitzgerald JC, Himebauch AS, et al. Clinical signs to categorize shock and target vasoactive medications in warm versus cold pediatric septic shock. Pediatr Crit Care Med. 2020;21(12):1051–8. https://doi.org/10.1097/PCC.0000000000002481. An interesting review of the clinical signs of shock type.
Peshimam N, Bruce-Hickman K, Crawford K, Upadhyay G, Randle E, Ramnarayan P, et al. Peripheral and central/intraosseous vasoactive infusions during and after pediatric critical care transport: retrospective cohort study of extravasation injury. Pediatr Crit Care Med. 2022;23(8):626–34. https://doi.org/10.1097/PCC.0000000000002972.
Owen VS, Rosgen BK, Cherak SJ, Ferland A, Stelfox HT, Fiest KM, et al. Adverse events associated with administration of vasopressor medications through a peripheral intravenous catheter: a systematic review and meta-analysis. Crit Care. 2021;25(1):146. https://doi.org/10.1186/s13054-021-03553-1. Published 2021 Apr 16.
Choong K, Bohn D, Fraser DD, Gaboury I, Hutchison JS, Joffe AR, et al. Vasopressin in pediatric vasodilatory shock: a multicenter randomized controlled trial. Am J Respir Crit Care Med. 2009;180(7):632–9. https://doi.org/10.1164/rccm.200902-0221OC.
Rich N, West N, McMaster P, Alexander J. Milrinone in meningococcal sepsis. Pediatr Crit Care Med. 2003;4(3):394–5. https://doi.org/10.1097/01.PCC.0000074278.30462.73.
Morin L, Ray S, Wilson C, Remy S, Benissa MR, Jansen NJG, et al. Refractory septic shock in children: a European Society of Paediatric and Neonatal Intensive Care definition. ESPNIC Refractory Septic Shock Definition Taskforce the Infection Systemic Inflammation Sepsis section of ESPNIC. Intensive Care Med. 2016;42(12):1948–57. https://doi.org/10.1007/s00134-016-4574-2.
Wolfler A, Calderini E, Iannella E, Conti G, Biban P, Dolcini A, et al. Evolution of noninvasive mechanical ventilation use: a cohort study among Italian PICUs. Pediatr Crit Care Med. 2015;16(5):418–27. https://doi.org/10.1097/PCC.0000000000000387.
Den Brinker M, Hokken-Koelega AC, Hazelzet JA, de Jong FH, Hop WC, Joosten KF. One single dose of etomidate negatively influences adrenocortical performance for at least 24h in children with meningococcal sepsis. Intensive Care Med. 2008;34(1):163–8. https://doi.org/10.1007/s00134-007-0836-3.
Godbout EJ, Pakyz AL, Markley JD, Noda AJ, Stevens MP. Pediatric antimicrobial stewardship: state of the art. Curr Infect Dis Rep. 2018;20(10):39. https://doi.org/10.1007/s11908-018-0644-7. Published 2018 Aug 1.
Lagunes L, Encina B, Ramirez-Estrada S. Current understanding in source control management in septic shock patients: a review. Ann Transl Med. 2016;4(17):330. https://doi.org/10.21037/atm.2016.09.02.
Gaines NN, Patel B, Williams EA, Cruz AT. Etiologies of septic shock in a pediatric emergency department population. Pediatr Infect Dis J. 2012;31(11):1203–5. https://doi.org/10.1097/INF.0b013e3182678ca9.
Lucignano B, Ranno S, Liesenfeld O, Pizzorno B, Putignani L, Bernaschi P, Menichella D. Multiplex PCR allows rapid and accurate diagnosis of bloodstream infections in newborns and children with suspected sepsis. J Clin Microbiol. 2011;49(6):2252–8. https://doi.org/10.1128/JCM.02460-10.
Ranjit S, Aram G, Kissoon N, Ali MK, Natraj R, Shresti S, et al. Multimodal monitoring for hemodynamic categorization and management of pediatric septic shock: a pilot observational study*. Pediatr Crit Care Med. 2014;15(1):e17–26. https://doi.org/10.1097/PCC.0b013e3182a5589c.
Goonasekera CDA, Carcillo JA, Deep A. Oxygen delivery and oxygen consumption in pediatric fluid refractory septic shock during the first 42 h of therapy and their relationship to 28-day outcome. Front Pediatr. 2018;23(6):314. https://doi.org/10.3389/fped.2018.00314.
Chen L, Li T, Fang F, Zhang Y, Faramand A. Tight glycemic control in critically ill pediatric patients: a systematic review and meta-analysis. Crit Care. 2018;22(1):57. https://doi.org/10.1186/s13054-018-1976-2. Published 2018 Mar 4.
Zimmerman JJ, Williams MD. Adjunctive corticosteroid therapy in pediatric severe sepsis: observations from the RESOLVE study. Pediatr Crit Care Med. 2011;12(1):2–8. https://doi.org/10.1097/PCC.0b013e3181d903f6.
Menon K, McNally D, Choong K, Sampson M. A systematic review and meta-analysis on the effect of steroids in pediatric shock. Pediatr Crit Care Med. 2013;14(5):474–80. https://doi.org/10.1097/PCC.0b013e31828a8125.
•• Nellis ME, Karam O, Valentine SL, Bateman ST, Remy KE, Lacroix J, et al. Executive summary of recommendations and expert consensus for plasma and platelet transfusion practice in critically Ill children: from the transfusion and anemia expertise initiative-control/avoidance of bleeding (TAXI-CAB). Pediatr Crit Care Med. 2022;23(1):34–51. https://doi.org/10.1097/PCC.0000000000002851. The most recent and complete society recommendations.
Sinitsky L, Walls D, Nadel S, Inwald DP. Fluid overload at 48 hours is associated with respiratory morbidity but not mortality in a general PICU: retrospective cohort study. Pediatr Crit Care Med. 2015;16(3):205–9. https://doi.org/10.1097/PCC.0000000000000318.
Miao H, Wang F, Xiong X, Wang C, Zhang Y. Clinical benefits of high-volume hemofiltration in critically Ill pediatric patients with severe sepsis: a retrospective cohort study. Blood Purif. 2018;45(1–3):18–27. https://doi.org/10.1159/000481249.
Khemani RG, Smith L, Lopez-Fernandez YM, Kwok J, Morzov R, Klein MJ, et al. Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study. Lancet Respir Med. 2019;7(2):115–28. https://doi.org/10.1016/S2213-2600(18)30344-8.
Kneyber MCJ, de Luca D, Calderini E, Jarreau PH, Javouhey E, Lopez-Herce J, et al. Recommendations for mechanical ventilation of critically ill children from the Paediatric Mechanical Ventilation Consensus Conference (PEMVECC). Intensive Care Med. 2017;43(12):1764–80. https://doi.org/10.1007/s00134-017-4920-z.
Macrae DJ, Field D, Mercier JC, Møller J, Stiris T, Biban P, et al. Inhaled nitric oxide therapy in neonates and children: reaching a European consensus. Intensive Care Med. 2004;30(3):372–80. https://doi.org/10.1007/s00134-003-2122-3.
• Melnikov G, Grabowski S, Broman LM. Extracorporeal membrane oxygenation for septic shock in children. ASAIO J. 2022;68(2):262–7. https://doi.org/10.1097/MAT.0000000000001464. An elegant review of the use of ECMO in septic shock.
Oberender F, Ganeshalingham A, Fortenberry JD, Hobson MJ, Houmes RJ, Morris KP, et al. Venoarterial extracorporeal membrane oxygenation versus conventional therapy in severe pediatric septic shock. Pediatr Crit Care Med. 2018;19(10):965–72.
Acknowledgements
The authors wish to thank Dr. Andrew Argent for reviewing their manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miranda, M., Nadel, S. Pediatric Sepsis: a Summary of Current Definitions and Management Recommendations. Curr Pediatr Rep 11, 29–39 (2023). https://doi.org/10.1007/s40124-023-00286-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40124-023-00286-3