Abstract
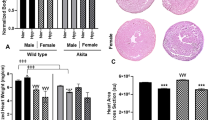
Hyperoxia, or supplemental oxygen, is regularly used in the clinical setting for critically ill patients in ICU. However, several recent studies have demonstrated the negative impact of this treatment in patients in critical care, including increased rates of lung and cardiac injury, as well as increased mortality. The purpose of this study was to determine the predisposition for arrhythmias and electrical remodeling in a type 2 diabetic mouse model (db/db), as a result of hyperoxia treatment. For this, db/db and their heterozygous controls were treated with hyperoxia (> 90% oxygen) or normoxia (normal air) for 72-h. Immediately following hyperoxia or normoxia treatments, mice underwent surface ECG. Excised left ventricles were used to assess ion channel expression, including for Kv1.4, Kv1.5, Kv4.2, and KChIP2. Serum cardiac markers were also measured, including cardiac troponin I and lactate dehydrogenase. Our results showed that db/db mice have increased sensitivity to arrhythmia. Normoxia-treated db/db mice displayed features of arrhythmia, including QTc and JT prolongation, as well as QRS prolongation. A significant increase in QRS prolongation was also observed in hyperoxia-treated db/db mice, when compared to hyperoxia-treated heterozygous control mice. Db/db mice were also shown to exhibit ion channel dysregulation, as demonstrated by down-regulation in Kv1.5, Kv4.2, and KChIP2 under hyperoxia conditions. From these results, we conclude that: (1) diabetic mice showed distinct pathophysiology, when compared to heterozygous controls, both in normoxia and hyperoxia conditions. (2) Diabetic mice were more susceptible to arrhythmia at normal air conditions; this effect was exacerbated at hyperoxia conditions. (3) Unlike in heterozygous controls, diabetic mice did not demonstrate cardiac hypertrophy as a result of hyperoxia. (4) Ion channel remodeling was also observed in db/db mice under hyperoxia condition similar to its heterozygous controls.





Similar content being viewed by others
References
Panguluri SK, Tur J, Chapalamadugu KC, Katnik C, Cuevas J, Tipparaju SM (2013) MicroRNA-301a mediated regulation of Kv4.2 in diabetes: identification of key modulators. PLoS ONE 8:e60545
Khavandi K, Khavandi A, Asghar O, Greenstein A, Withers S, Heagerty AM, Malik RA (2009) Diabetic cardiomyopathy—a distinct disease? Best Pract Res Clin Endocrinol Metab 23:347–360
Barth AS, Tomaselli GF (2009) Cardiac metabolism and arrhythmias. Circ Arrhythm Electrophysiol 2:327–335
Ozturk N, Olgar Y, Ozdemir S (2013) Trace elements in diabetic cardiomyopathy: an electrophysiological overview. World J Diabetes 4:92–100
Morita H, Watanabe A, Morimoto Y, Kawada S, Tachibana M, Nakagawa K, Nishii N, Ito H (2017) Distribution and prognostic significance of fragmented QRS in patients with Brugada syndrome. Circ Arrhythm Electrophysiol 10(3):e004765
Steger A, Sinnecker D, Berkefeld A, Muller A, Gebhardt J, Dommasch M, Huster KM, Barthel P, Schmidt G (2015) Fragmented QRS. Relevance in clinical practice. Herzschrittmacherther Elektrophysiol 26:235–241
Nishiyama A, Ishii DN, Backx PH, Pulford BE, Birks BR, Tamkun MM (2001) Altered K(+) channel gene expression in diabetic rat ventricle: isoform switching between Kv4.2 and Kv1.4. Am J Physiol Heart Circ Physiol 281:H1800–H1807
Li X, Xu Z, Li S, Rozanski GJ (2005) Redox regulation of Ito remodeling in diabetic rat heart. Am J Physiol Heart Circ Physiol 288:H1417–H1424
Sato T, Kobayashi T, Kuno A, Miki T, Tanno M, Kouzu H, Itoh T, Ishikawa S, Kojima T, Miura T, Tohse N (2014) Type 2 diabetes induces subendocardium-predominant reduction in transient outward K+ current with downregulation of Kv4.2 and KChIP2. Am J Physiol Heart Circ Physiol 306:H1054–H1065
Panguluri SK, Tur J, Fukumoto J, Deng W, Sneed KB, Kolliputi N, Bennett ES, Tipparaju SM (2013) Hyperoxia-induced hypertrophy and ion channel remodeling in left ventricle. Am J Physiol Heart Circ Physiol 304:H1651–H1661
Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM (2010) The epidemiology of mechanical ventilation use in the United States. Crit Care Med 38:1947–1953
Hovaguimian F, Lysakowski C, Elia N, Tramer M (2013) Effect of intraoperative high inspired oxygen fraction on surgical site infection, postoperative nausea and vomiting, and pulmonary function: systematic review and meta-analysis of randomized controlled trials. Anesthesiology 119:303–316
Iscoe S, Beasley R, Fisher JA (2011) Supplementary oxygen for nonhypoxemic patients: O2 much of a good thing? Crit Care 15:305
Johnston AJ, Steiner LA, Gupta AK, Menon DK (2003) Cerebral oxygen vasoreactivity and cerebral tissue oxygen reactivity. Br J Anaesth 90:774–786
Floyd TF, Clark JM, Gelfand R, Detre JA, Ratcliffe S, Guvakov D, Lambertsen CJ, Eckenhoff RG (2003) Independent cerebral vasoconstrictive effects of hyperoxia and accompanying arterial hypocapnia at 1 ATA. J Appl Physiol 95:2453–2461
Farquhar H, Weatherall M, Wijesinghe M, Perrin K, Ranchord A, Simmonds M, Beasley R (2009) Systematic review of studies of the effect of hyperoxia on coronary blood flow. Am Heart J158:371–377
Kutsogiannis DJ, Bagshaw SM, Laing B, Brindley PG (2011) Predictors of survival after cardiac or respiratory arrest in critical care units. CMAJ 183:1589–1595
Damiani E, Adrario E, Girardis M, Romano R, Pelaia P, Singer M, Donati A (2014) Arterial hyperoxia and mortality in critically ill patients: a systematic review and meta-analysis. Crit Care 18:711
Helmerhorst HJF, Schultz MJ, van der Voort PHJ, Bosman RJ, Juffermans NP, de Jonge E, van Westerloo DJ (2014) Self-reported attitudes versus actual practice of oxygen therapy by ICU physicians and nurses. Ann Intensive Care 4:23
Kilgannon JH, Jones AE, Shapiro NI, Angelos MG, Milcarek B, Hunter K, Parrillo JE, Trzeciak S, Emergency Medicine Shock Research Network, I (2010) Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA 303:2165–2171
Rincon F, Kang J, Vibbert M, Urtecho J, Athar MK, Jallo J (2014) Significance of arterial hyperoxia and relationship with case fatality in traumatic brain injury: a multicentre cohort study. J Neurol Neurosurg Psychiatry 85:799–805
Rincon F, Kang J, Maltenfort M, Vibbert M, Urtecho J, Athar MK, Jallo J, Pineda CC, Tzeng D, McBride W, Bell R (2014) Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit Care Med 42:387–396
Nelskyla A, Parr MJ, Skrifvars MB (2013) Prevalence and factors correlating with hyperoxia exposure following cardiac arrest–an observational single centre study. Scand J Trauma Resusc Emerg Med 21:35
Kallet R, Matthay MA (2013) Hyperoxic acute lung injury. Respir Care 58:123–141
Xu D, Guthrie JR, Mabry S, Sack TM, Truog WE (2006) Mitochondrial aldehyde dehydrogenase attenuates hyperoxia-induced cell death through activation of ERK/MAPK and PI3K-Akt pathways in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 291:L966–L975
Shosholcheva M, Jankulovski N, Kartalov A, Kuzmanovska B, Miladinova D (2017) Synergistic effect of hyperoxia and biotrauma on ventilator-induced lung injury. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 38:91–96
Visser YP, Walther FJ, el Laghmani H, Laarse A, Wagenaar GT (2010) Apelin attenuates hyperoxic lung and heart injury in neonatal rats. Am J Respir Crit Care Med 182:1239–1250
Gole Y, Gargne O, Coulange M, Steinberg JG, Bouhaddi M, Jammes Y, Regnard J, Boussuges A (2011) Hyperoxia-induced alterations in cardiovascular function and autonomic control during return to normoxic breathing. Eur J Appl Physiol 111:937–946
Seals DR, Johnson DG, Fregosi RF (1991) Hyperoxia lowers sympathetic activity at rest but not during exercise in humans. Am J Physiol 260:R873–R878
Waring WS, Thomson AJ, Adwani SH, Rosseel AJ, Potter JF, Webb DJ, Maxwell SR (2003) Cardiovascular effects of acute oxygen administration in healthy adults. J Cardiovasc Pharmacol 42:245–250
Chapalamadugu KC, Pangulur SK, Bennett ES, Kolliputi N, Tipparaju SM (2015) High level of oxygen treatment causes cardiotoxicity with arrhythmias and redox modulation. Toxicol Appl Pharmacol 282:100–107
Rezende PC, Rahmi RM, Uchida AH, da Costa LM, Scudeler TL, Garzillo CL, Lima EG, Segre CA, Girardi P, Takiuti M, Silva MF, Hueb W, Ramires JA, Kalil Filho R (2015) Type 2 diabetes mellitus and myocardial ischemic preconditioning in symptomatic coronary artery disease patients. Cardiovasc Diabetol 14:66
Vinokur V, Berenshtein E, Bulvik B, Grinberg L, Eliashar R, Chevion M (2013) The bitter fate of the sweet heart: impairment of iron homeostasis in diabetic heart leads to failure in myocardial protection by preconditioning. PLoS ONE 8:e62948
Vogel WM, Apstein CS (1988) Effects of alloxan-induced diabetes on ischemia-reperfusion injury in rabbit hearts. Circ Res 62:975–982
Forrat R, Sebbag L, Wiernsperger N, Guidollet J, Renaud S, de Lorgeril M (1993) Acute myocardial infarction in dogs with experimental diabetes. Cardiovasc Res 27:1908–1912
Liu Y, Thornton JD, Cohen MV, Downey JM, Schaffer SW (1993) Streptozotocin-induced non-insulin-dependent diabetes protects the heart from infarction. Circulation 88:1273–1278
Roglic G, Unwin N, Bennett PH, Mathers C, Tuomilehto J, Nag S, Connolly V, King H (2005) The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care 28:2130–2135
Stahn A, Pistrosch F, Ganz X, Teige M, Koehler C, Bornstein S, Hanefeld M (2014) Relationship between hypoglycemic episodes and ventricular arrhythmias in patients with type 2 diabetes and cardiovascular diseases: silent hypoglycemias and silent arrhythmias. Diabetes Care 37(2):516–520
Pistrosch F, Ganz X, Bornstein SR, Birkenfeld AL, Henkel E, Hanefeld M (2015) Risk of and risk factors for hypoglycemia and associated arrhythmias in patients with type 2 diabetes and cardiovascular disease: a cohort study under real-world conditions. Acta Diabetol 52(5):889–895
Kato T, Yamashita T, Sekiguchi A, Sagara K, Takamura M, Takata S, Kaneko S, Aizawa T, Fu LT (2006) What are arrhythmogenic substrates in diabetic rat atria? J Cardiovasc Electrophysiol 17(8):890–894
Kato T, Yamashita T, Sekiguchi A, Tsuneda T, Sagara K, Takamura M, Kaneko S, Aizawa T, Fu LT (2008) AGEs-RAGE system mediates atrial structural remodeling in the diabetic rat. J Cardiovasc Electrophysiol 19(4):415–420
Farag YM, Gaballa MR (2011) Diabesity: an overview of a rising epidemic. Nephrol Dial Transplant 26:28–35
Huynh K, Bernardo BC, McMullen JR, Ritchie RH (2014) Diabetic cardiomyopathy: mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol Ther 142:375–415
Feng B, Chen S, Chiu J, George B, Chakrabarti S (2008) Regulation of cardiomyocyte hypertrophy in diabetes at the transcriptional level. Am J Physiol Endocrinol Metab 294:E1119–E1126
Soltysinska E, Speerschneider T, Winther SV, Thomsen MB (2014) Sinoatrial node dysfunction induces cardiac arrhythmias in diabetic mice. Cardiovasc Diabetol 13:122
Venardos K, De Jong KA, Elkamie M, Connor T, McGee SL (2015) The PKD inhibitor CID755673 enhances cardiac function in diabetic db/db mice. PLoS ONE 10:e0120934
Hall ME, Maready MW, Hall JE, Stec DE (2014) Rescue of cardiac leptin receptors in db/db mice prevents myocardial triglyceride accumulation. Am J Physiol Endocrinol Metab 307:E316–E325
Dalbøge LS, Almholt DLC, Neerup TSR, Vassiliadis E, Vrang N, Pedersen L, Fosgerau K, Jelsing J (2013) Characterisation of age-dependent beta cell dynamics in the male db/db mice. PLoS ONE 8:e82813
Kawasaki F, Matsuda M, Kanda Y, Inoue H, Kaku K (2005) Structural and functional analysis of pancreatic islets preserved by pioglitazone in db/db mice. Am J Physiol Endocrinol Metab 288:E510–E518
Hadour G, Ferrera R, Sebbag L, Forrat R, Delaye J, de Lorgeril M (1998) Improved myocardial tolerance to ischaemia in the diabetic rabbit. J Mol Cell Cardiol 30:1869–1875
Crow RS, Hannan PJ, Folsom AR (2003) Prognostic significance of corrected QT and corrected JT interval for incident coronary heart disease in a general population sample stratified by presence or absence of wide QRS complex: the ARIC Study with 13 years of follow-up. Circulation 108:1985–1989
Yan GX, Antzelevitch C (1998) Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation 98:1928–1936
Tse G, Lai ET, Tse V, Yeo JM (2016) Molecular and electrophysiological mechanisms underlying cardiac arrhythmogenesis in diabetes mellitus. J Diabetes Res 2016:2848759
Hasslacher C, Wahl P (1977) Diabetes prevalence in patients with bradycardiac arrhythmias. Acta Diabetol Lat 14:229–234
Movahed MR, Hashemzadeh M, Jamal MM (2005) Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardiovascular disease. Int J Cardiol 105:315–318
Meek S, Morris F (2002) ABC of clinical electrocardiography. Introduction. I—leads, rate, rhythm, and cardiac axis. BMJ 324(7334):415–418
Goncalves AC, Tank J, Diedrich A, Hilzendeger A, Plehm R, Bader M, Luft FC, Jordan J, Gross V (2009) Diabetic hypertensive leptin receptor-deficient db/db mice develop cardioregulatory autonomic dysfunction. Hypertension 53:387–392
Senador D, Kanakamedala K, Irigoyen MC, Morris M, Elased KM (2009) Cardiovascular and autonomic phenotype of db/db diabetic mice. Exp Physiol 94:648–658
Schaan BD, Dall’Ago P, Maeda CY, Ferlin E, Fernandes TG, Schmid H, Irigoyen MC (2004) Relationship between cardiovascular dysfunction and hyperglycemia in streptozotocin-induced diabetes in rats. Braz J Med Biol Res 37:1895–1902
De Angelis K, Schaan BD, Maeda CY, Dall’Ago P, Wichi RB, Irigoyen MC (2002) Cardiovascular control in experimental diabetes. Braz J Med Biol Res 35:1091–1100
Baslaib F, Alkaabi S, Yan AT, Yan RT, Dorian P, Nanthakumar K, Casanova A, Goodman SG, Canadian Acute Coronary Syndrome Registry I (2010) QRS prolongation in patients with acute coronary syndromes. Am Heart J 159:593–598
Kurl S, Makikallio TH, Rautaharju P, Kiviniemi V, Laukkanen JA (2012) Duration of QRS complex in resting electrocardiogram is a predictor of sudden cardiac death in men. Circulation 125:2588–2594
Deen JF, Rhoades DA, Noonan C, Best LG, Okin PM, Devereux RB, Umans JG (2017) Comparison of QRS duration and associated cardiovascular events in American Indian men versus women (The Strong Heart Study). Am J Cardiol 119:1757–1762
Zhou Y, Jelinek H, Hambly BD, McLachlan CS (2017) Electrocardiogram QRS duration and associations with telomere length: a cross-sectional analysis in Australian rural diabetic and non-diabetic population. J Electrocardiol 50:450–456
O’Brien PJ, Smith DE, Knechtel TJ, Marchak MA, Pruimboom-Brees I, Brees DJ, Spratt DP, Archer FJ, Butler P, Potter AN, Provost JP, Richard J, Snyder PA, Reagan WJ (2006) Cardiac troponin I is a sensitive, specific biomarker of cardiac injury in laboratory animals. Lab Anim 40:153–171
Bagi Z, Erdei N, Toth A, Li W, Hintze TH, Koller A, Kaley G (2005) Type 2 diabetic mice have increased arteriolar tone and blood pressure: enhanced release of COX-2-derived constrictor prostaglandins. Arterioscler Thromb Vasc Biol 25:1610–1616
Casis O, Gallego M, Iriarte M, Sanchez-Chapula JA (2000) Effects of diabetic cardiomyopathy on regional electrophysiologic characteristics of rat ventricle. Diabetologia 43:101–109
Nerbonne JM, Kass RS (2005) Molecular physiology of cardiac repolarization. Physiol Rev 85:1205–1253
Gidh-Jain M, Huang B, Jain P, El-Sherif N (1996) Differential expression of voltage-gated K+ channel genes in left ventricular remodeled myocardium after experimental myocardial infarction. Circ Res 79:669–675
Kaprielian R, Wickenden AD, Kassiri Z, Parker TG, Liu PP, Backx PH (1999) Relationship between K+ channel down-regulation and [Ca2+]i in rat ventricular myocytes following myocardial infarction. J Physiol 517(Pt 1):229–245
An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ (2000) Modulation of A-type potassium channels by a family of calcium sensors. Nature 403:553–556
Wang Y, Xu H, Kumar R, Tipparaju SM, Wagner MB, Joyner RW (2003) Differences in transient outward current properties between neonatal and adult human atrial myocytes. J Mol Cell Cardiol 35:1083–1092
Suzuki T, Shioya T, Murayama T, Sugihara M, Odagiri F, Nakazato Y, Nishizawa H, Chugun A, Sakurai T, Daida H, Morimoto S, Kurebayashi N (2012) Multistep ion channel remodeling and lethal arrhythmia precede heart failure in a mouse model of inherited dilated cardiomyopathy. PLoS ONE 7:e35353
Dartsch T, Fischer R, Gapelyuk A, Weiergraeber M, Ladage D, Schneider T, Schirdewan A, Reuter H, Mueller-Ehmsen J, Zobel C (2013) Aldosterone induces electrical remodeling independent of hypertension. Int J Cardiol 164:170–178
Kuo HC, Cheng CF, Clark RB, Lin JJ, Lin JL, Hoshijima M, Nguyen-Tran VT, Gu Y, Ikeda Y, Chu PH, Ross J, Giles WR, Chien KR (2001) A defect in the Kv channel-interacting protein 2 (KChIP2) gene leads to a complete loss of I(to) and confers susceptibility to ventricular tachycardia. Cell 107:801–813
Acknowledgements
We thank Dr. Kolliputi for giving us access to qRT-PCR instrument and hyperoxia chamber. We also thank Dr. Noujaim for his advice for this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Rodgers, J.L., Samal, E., Mohapatra, S. et al. Hyperoxia-induced cardiotoxicity and ventricular remodeling in type-II diabetes mice. Heart Vessels 33, 561–572 (2018). https://doi.org/10.1007/s00380-017-1100-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-017-1100-6