Abstract
Oxygen plays a central role in cardiac energy metabolism. At high altitude where the ambient oxygen level is low, we found EDNRB is associated with human hypoxia adaptation. Our subsequent study in global heterozygous knockout mice (Ednrb−/+) revealed that cardiac function was conserved in these mice when exposed to extreme hypoxia. The major goal of this study was (i) to determine the functional role of cardiomyocyte EdnrB in maintaining cardiac function under hypoxic stress and (ii) to validate the phenotypes we detected in Ednrb−/+ mice using EDNRB blockers. Unlike the global knockouts, cardiac-specific heterozygote (EdnrBflox/+) and homozygote (EdnrBflox/flox) EdnrB knockout mice were phenotypically normal. When treated with graded low levels of oxygen (10% and 5% O2), both EdnrBflox/+ and EdnrBflox/flox were hypoxia tolerant. The cardiac indexes at 10% and 5% O2 for EdnrBflox/+ were significantly higher and lactate levels were significantly lower when compared to the cre-negative controls (P < 0.05). Simultaneously, mice treated with BQ-788 (EDNRB-specific blocker) had a significantly higher cardiac index (P < 0.005) and significantly lower lactate levels (P < 0.0001) than in control mice. A similar result was obtained with mice treated with Bosentan (non-specific). These data indicate that a lower level or complete lack of EdnrB in the cardiomyocytes significantly improves cardiac performance under extreme hypoxia, a novel role of cardiomyocyte EdnrB in the regulation of cardiac function. Furthermore, this rescue under extreme hypoxia can also be achieved using EDNRB-specific pharmacological agents, e.g., BQ-788. This systematically confirms, both genetically and pharmacologically, the protective role of a lower EDNRB under extreme hypoxia stress.
Key messages
-
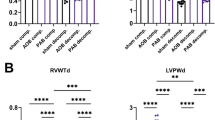
Under normal condition, cardiomyocytes-specific EdnrB knockout mice, both heterozygote and homozygote, are phenotypically normal.
-
Under hypoxic condition, a lower level or complete deletion of cardiomyocyte EdnrB conserves cardiac function by maintaining high cardiac index.
-
Similarly, mice treated with both specific (BQ-788) and non-specific (Bosentan) EDNRB blockers are tolerant to hypoxia by maintaining better cardiac function.
-
The oxygen perfusion under extreme hypoxia is better in the mice with lower EDNRB, as depicted by lower lactate level at 5% oxygen.
-
Our current study systematically confirms, both genetically and pharmacologically, the protective role of a lower EDNRB under extreme hypoxia stress.
-
Overall, it supports our hypothesis that studies on human hypoxia adaptation provide new insight to common disease pathogenesis and treatments.




Similar content being viewed by others

References
Udpa N, Ronen R, Zhou D, Liang J, Stobdan T, Appenzeller O, Yin Y, du Y, Guo L, Cao R et al (2014) Whole genome sequencing of Ethiopian highlanders reveals conserved hypoxia tolerance genes. Genome Biol 15(2):R36
Zhou D, Udpa N, Ronen R, Stobdan T, Liang J, Appenzeller O, Zhao HW, Yin Y, Du Y, Guo L et al (2013) Whole-genome sequencing uncovers the genetic basis of chronic mountain sickness in Andean highlanders. Am J Hum Genet 93(3):452–462
Stobdan T, Zhou D, Ao-Ieong E, Ortiz D, Ronen R, Hartley I, Gan Z, McCulloch AD, Bafna V, Cabrales P et al (2015) Endothelin receptor B, a candidate gene from human studies at high altitude, improves cardiac tolerance to hypoxia in genetically engineered heterozygote mice. Proc Natl Acad Sci U S A 112(33):10425–10430
Naeije R (2010) Physiological adaptation of the cardiovascular system to high altitude. Prog Cardiovasc Dis 52(6):456–466
Goerre S, Wenk M, Bartsch P, Luscher TF, Niroomand F, Hohenhaus E, Oelz O, Reinhart WH (1995) Endothelin-1 in pulmonary hypertension associated with high-altitude exposure. Circulation 91(2):359–364
Kojonazarov B, Isakova J, Imanov B, Sovkhozova N, Sooronbaev T, Ishizaki T, Aldashev AA (2012) Bosentan reduces pulmonary artery pressure in high altitude residents. High Alt Med Biol 13(3):217–223
Kuc RE, Maguire JJ, Davenport AP (2006) Quantification of endothelin receptor subtypes in peripheral tissues reveals downregulation of ET(A) receptors in ET(B)-deficient mice. Exp Biol Med (Maywood) 231(6):741–745
Kedzierski RM, Grayburn PA, Kisanuki YY, Williams CS, Hammer RE, Richardson JA, Schneider MD, Yanagisawa M (2003) Cardiomyocyte-specific endothelin A receptor knockout mice have normal cardiac function and an unaltered hypertrophic response to angiotensin II and isoproterenol. Mol Cell Biol 23(22):8226–8232
Krejci V, Hiltebrand LB, Erni D, Sigurdsson GH (2003) Endothelin receptor antagonist bosentan improves microcirculatory blood flow in splanchnic organs in septic shock. Crit Care Med 31(1):203–210
Wanecek M, Weitzberg E, Alving K, Rudehill A, Oldner A (2001) Effects of the endothelin receptor antagonist bosentan on cardiac performance during porcine endotoxin shock. Acta Anaesthesiol Scand 45(10):1262–1270
Bagnall AJ, Kelland NF, Gulliver-Sloan F, Davenport AP, Gray GA, Yanagisawa M, Webb DJ, Kotelevtsev YV (2006) Deletion of endothelial cell endothelin B receptors does not affect blood pressure or sensitivity to salt. Hypertension 48(2):286–293
Druckenbrod NR, Powers PA, Bartley CR, Walker JW, Epstein ML (2008) Targeting of endothelin receptor-B to the neural crest. Genesis 46(8):396–400
Cabrales P, Acero C, Intaglietta M, Tsai AG (2003) Measurement of the cardiac output in small animals by thermodilution. Microvasc Res 66(2):77–82
Dimitrijevic I, Edvinsson ML, Chen Q, Malmsjo M, Kimblad PO, Edvinsson L (2009) Increased expression of vascular endothelin type B and angiotensin type 1 receptors in patients with ischemic heart disease. BMC Cardiovasc Disord 9:40
Dagassan PH, Breu V, Clozel M, Kunzli A, Vogt P, Turina M et al (1996) Up-regulation of endothelin-B receptors in atherosclerotic human coronary arteries. J Cardiovasc Pharmacol 27(1):147–153
Tykocki NR, Watts SW (2010) The interdependence of endothelin-1 and calcium: a review. Clin Sci (Lond) 119(9):361–372
Schneider MP, Boesen EI, Pollock DM (2007) Contrasting actions of endothelin ET(A) and ET(B) receptors in cardiovascular disease. Annu Rev Pharmacol Toxicol 47:731–759
Lee HO, Levorse JM, Shin MK (2003) The endothelin receptor-B is required for the migration of neural crest-derived melanocyte and enteric neuron precursors. Dev Biol 259(1):162–175
Hosoda K, Hammer RE, Richardson JA, Baynash AG, Cheung JC, Giaid A, Yanagisawa M (1994) Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell 79(7):1267–1276
Gariepy CE, Williams SC, Richardson JA, Hammer RE, Yanagisawa M (1998) Transgenic expression of the endothelin-B receptor prevents congenital intestinal aganglionosis in a rat model of Hirschsprung disease. J Clin Invest. 102(6):1092–1101
Ge Y, Bagnall A, Stricklett PK, Strait K, Webb DJ, Kotelevtsev Y, Kohan DE (2006) Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am J Physiol Renal Physiol 291(6):F1274–F1280
Kelland NF, Kuc RE, McLean DL, Azfer A, Bagnall AJ, Gray GA et al (2010) Endothelial cell-specific ETB receptor knockout: autoradiographic and histological characterisation and crucial role in the clearance of endothelin-1. Can J Physiol Pharmacol 88(6):644–651
Fukuroda T, Fujikawa T, Ozaki S, Ishikawa K, Yano M, Nishikibe M (1994) Clearance of circulating endothelin-1 by ETB receptors in rats. Biochem Biophys Res Commun 199(3):1461–1465
Miller E, Czopek A, Duthie KM, Kirkby NS, van de Putte EE, Christen S et al (2017) Smooth muscle endothelin B receptors regulate blood pressure but not vascular function or neointimal remodeling. Hypertension. 69(2):275–285
Zhao XS, Pan W, Bekeredjian R, Shohet RV (2006) Endogenous endothelin-1 is required for cardiomyocyte survival in vivo. Circulation 114(8):830–837
Merlen C, Farhat N, Luo X, Chatenet D, Tadevosyan A, Villeneuve LR, Gillis MA, Nattel S, Thorin E, Fournier A (2013) et al. Intracrine endothelin signaling evokes IP3-dependent increases in nucleoplasmic Ca(2)(+) in adult cardiac myocytes. J Mol Cell Cardiol 62:189–202
Jules F, Avedanian L, Al-Khoury J, Keita R, Normand A, Bkaily G et al (2015) Nuclear membranes ETB receptors mediate ET-1-induced increase of nuclear calcium in human left ventricular endocardial endothelial cells. J Cardiovasc Pharmacol 66(1):50–57
Pham I, Wuerzner G, Richalet JP, Peyrard S, Azizi M (2010) Endothelin receptors blockade blunts hypoxia-induced increase in PAP in humans. Eur J Clin Investig 40(3):195–202
Radiloff DR, Zhao Y, Boico A, Wu C, Shan S, Palmer G et al (2012) The combination of theophylline and endothelin receptor antagonism improves exercise performance of rats under simulated high altitude. J Appl Physiol (1985) 113(8):1243–1252
Schroeder T, Piantadosi CA, Natoli MJ, Autmizguine J, Cohen-Wolkowieczs M, Hamilton KL et al (2018) Safety and ergogenic properties of combined aminophylline and ambrisentan in hypoxia. Clin Pharmacol Ther. 103(5):888–898
Naeije R, Huez S, Lamotte M, Retailleau K, Neupane S, Abramowicz D, Faoro V (2010) Pulmonary artery pressure limits exercise capacity at high altitude. Eur Respir J 36(5):1049–1055
Beall CM, Decker MJ, Brittenham GM, Kushner I, Gebremedhin A, Strohl KP (2002) An Ethiopian pattern of human adaptation to high-altitude hypoxia. Proc Natl Acad Sci U S A 99(26):17215–17218
Wu S, Hao G, Zhang S, Jiang D, Wuren T, Luo J (2016) Cerebral vasoconstriction reactions and plasma levels of ETBR, ET-1, and eNOS in patients with chronic high altitude disease. Mol Med Rep. 14(3):2497–2502
Georgiadis D, Sievert M, Cencetti S, Uhlmann F, Krivokuca M, Zierz S, Werdan K (2000) Cerebrovascular reactivity is impaired in patients with cardiac failure. Eur Heart J 21(5):407–413
Foller M, Mahmud H, Qadri SM, Gu S, Braun M, Bobbala D et al (2010) Endothelin B receptor stimulation inhibits suicidal erythrocyte death. FASEB J 24(9):3351–3359
Shihoya W, Nishizawa T, Okuta A, Tani K, Dohmae N, Fujiyoshi Y, Nureki O, Doi T (2016) Activation mechanism of endothelin ETB receptor by endothelin-1. Nature 537(7620):363–368
Acknowledgements
We thank Dr. Donald Kohan (Univ. of Utah) and Dr. David Webb (Univ. Of Edinburgh) for providing the EdnrB-LoxP mice.
Sources of funding
This work was supported by National Institutes of Health (NIH) grant (R01 HL127403-02) to GGH. TS was supported by the Emerald Foundation Inc. This work was partially supported by NIH grants from the Heart Lung and Blood Institute (P01-HL110900, R01-HL126945, and T32-HL007444) PC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Fig S1
Cardiac output comparison between cardiac-specific EdnrB homozygous (EdnrBflox/flox), heterozygous (EdnrBflox/+) and controls mice. EdnrBflox/+ mice maintains a higher CO under hypoxia. Error bar indicates ± standard error.(PNG 73 kb)
Supplementary Fig S2
Cardiac output comparison between BQ-788, Bosentan treated and control mice. Both BQ-788 and Bosentan treated mice maintains a relative higher CO under extreme hypoxia of 10% and 5% O2. However,CO in BQ-788 treated mice was significantly higher at 5% O2 when compared to both Bosentan and control mice. Error bar indicates ± standard error.(PNG 50 kb)
Rights and permissions
About this article
Cite this article
Stobdan, T., Zhou, D., Williams, A.T. et al. Cardiac-specific knockout and pharmacological inhibition of Endothelin receptor type B lead to cardiac resistance to extreme hypoxia. J Mol Med 96, 975–982 (2018). https://doi.org/10.1007/s00109-018-1673-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-018-1673-2