Abstract
Introduction
The safety of arterial hyperoxia is under increasing scrutiny. We performed a systematic review of the literature to determine whether any association exists between arterial hyperoxia and mortality in critically ill patient subsets.
Methods
Medline, Thomson Reuters Web of Science and Scopus databases were searched from inception to June 2014. Observational or interventional studies evaluating the relationship between hyperoxia (defined as a supranormal arterial O2 tension) and mortality in adult intensive care unit (ICU) patients were included. Studies primarily involving patients with exacerbations of chronic pulmonary disease, acute lung injury and perioperative administration were excluded. Adjusted odds ratio (OR) of patients exposed versus those not exposed to hyperoxia were extracted, if available. Alternatively, unadjusted outcome data were recorded. Data on patients, study characteristics and the criteria used for defining hyperoxia exposure were also extracted. Random-effects models were used for quantitative synthesis of the data, with a primary outcome of hospital mortality.
Results
In total 17 studies (16 observational, 1 prospective before-after) were identified in different patient categories: mechanically ventilated ICU (number of studies (k) = 4, number of participants (n) = 189,143), post-cardiac arrest (k = 6, n = 19,144), stroke (k = 2, n = 5,537), and traumatic brain injury (k = 5, n = 7,488). Different criteria were used to define hyperoxia in terms of PaO2 value (first, highest, worst, mean), time of assessment and predetermined cutoffs. Data from studies on ICU patients were not pooled because of extreme heterogeneity (inconsistency (I2) 96.73%). Hyperoxia was associated with increased mortality in post-cardiac arrest patients (OR = 1.42 (1.04 to 1.92) I2 67.73%) stroke (OR = 1.23 (1.06 to 1.43) I2 0%) and traumatic brain injury (OR = 1.41 (1.03 to 1.94) I2 64.54%). However, these results are limited by significant heterogeneity between studies.
Conclusions
Hyperoxia may be associated with increased mortality in patients with stroke, traumatic brain injury and those resuscitated from cardiac arrest. However, these results are limited by the high heterogeneity of the included studies.
Similar content being viewed by others
Introduction
Oxygen (O2) administration is the most widely prescribed therapy in critically ill patients and frequently represents a life-saving intervention. Since hypoxemia is generally viewed as deleterious and moderate levels of arterial hyperoxia as benign, health care practitioners are more likely to accept supranormal arterial O2 levels as this provides a wider safety buffer [1],[2].
The use of supplemental O2 in various medical emergencies is supported by many guidelines [3]-[5]. One hundred percent O2 is commonly administered during cardiopulmonary resuscitation from cardiac arrest [6]. Normobaric hyperoxia is touted as a potential therapeutic strategy for patients with traumatic brain injury or stroke [7], with an underlying rationale of increased brain O2 delivery [8] and protection of the ischemic penumbra through inducing redistribution of blood from normal to ischemic areas [9]. However, these potential benefits must be weighed against the injurious effects of high-dose supplemental O2. In both animal and human studies there are reports of pulmonary toxicity [10]-[12], increased vasoconstriction with a fall in cardiac output [13], free radical-mediated damage to various organs [14], and a marked reduction in coronary blood flow and myocardial O2 consumption [15].
Clinical data regarding the relationship between arterial hyperoxia and outcome are contradictory. An association between arterial hyperoxia and mortality has been reported in disparate patient populations (mechanically ventilated [16], post-cardiac arrest [17], traumatic brain injury [18], stroke [19]) but not confirmed by other studies [20]-[23]. Therefore, the question whether exposure to supranormal arterial O2 tensions (PaO2) is safe in critically ill patients remains unanswered. We thus performed a systematic review and meta-analysis of studies describing the relationship between arterial hyperoxia and mortality in critically ill patients.
Materials and methods
This report adheres to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) standards for reporting systematic review and meta-analysis studies [24].
Eligibility criteria
Observational (prospective or retrospective cohort or case-control studies) or randomized controlled trials (RCTs) investigating the relationship between arterial hyperoxia and mortality in critically ill patients were eligible for inclusion. Participants were required to be adult patients admitted to a critical care unit for any reason. We excluded studies involving patients with an acute exacerbation of chronic obstructive pulmonary disease (COPD) or acute lung injury (ALI). As hypoxemia is the main problem in these patients, studies on the impact of hyperoxemia were likely to be uncommon. We expected that studies on patients with COPD/ALI may have explored the effects of excessive O2 flow rather than those of arterial hyperoxemia. In addition, in these patients the PaO2 range defined as normal/acceptable could have been lower than that applied in patients with preserved respiratory function. Studies on surgical patients were excluded unless they were exposed to hyperoxia during a post-operative admission to a critical care unit. Hyperoxia was defined by the measurement of supranormal values of arterial partial O2 pressure (PaO2). For defining a condition of exposure to hyperoxia, any cutoff value of PaO2 and time of assessment were deemed acceptable. Studies where patients were defined as ‘hyperoxic’ solely on the basis of exposure to a predetermined increase in inspired O2 fraction (FiO2) were excluded if not guided by any assessment of PaO2. Patients not exposed to hyperoxia constituted the comparator group. The primary outcome of interest was hospital mortality from any cause.
Search strategy
Studies were identified by searching Medline (PubMed), Scopus and Thomson Reuters Web of Science databases from their inception. The main search was run on 28 March 2014 and updated weekly until June 2014. The keywords ‘hyperoxia’, ‘hyperoxemia’, ‘arterial oxygen’, ‘oxygen saturation’, ‘critically ill’, ‘acutely ill’, ‘intensive care’, ‘critical care’, ‘mechanically ventilated’, ‘cardiac arrest’, ‘cardiopulmonary resuscitation’, ‘traumatic brain injury’, ‘head trauma’, ‘stroke’, ‘sepsis’, ‘septic shock’, ‘trauma’, ‘post-operative’, ‘post-surgery’, ‘cardiac failure’, ‘heart failure’, ‘myocardial infarction’, ‘shock’, ‘mortality’, ‘survival’, ‘death’, ‘outcome’ were typed in various combinations using Boolean operators. Queries were limited to those involving human subjects. The detailed search strategy applied to Medline (PubMed), and adapted for the other databases, is described in Additional file 1. Hand searches of reference lists of articles and relevant literature reviews were used to complement the computer search. Content pages of the main critical care medicine journals were hand-searched to find any relevant in-press articles. The search was not limited by language, but focused solely on articles published in peer-reviewed journals to enhance the methodological rigor of the studies examined and the conclusions drawn.
Study selection
Two independent reviewers (ED and EA) screened all identified records (title and abstract) and performed the eligibility assessment of the selected full-text articles in an unblinded standardized manner. Disagreements were resolved through discussion or arbitration by a third reviewer (AD). Interobserver agreement was assessed by kappa statistics.
Data extraction
Two independent investigators (ED and EA) extracted descriptive, methodological and outcome data from all eligible studies using a predefined data extraction form. Disagreements were resolved through consensus. The datasheet included study design (RCT, retrospective or prospective observational study, multicenter or single-center), country, years of enrollment, publication year, primary endpoint, sample size, mean age (as a continuous variable), gender distribution (as a percentage of males), category of critical illness (mechanically ventilated, post-cardiac arrest, traumatic brain injury, stroke, other), criteria for the definition of hyperoxia exposure (time of assessment, selection of the first/highest/worst/mean PaO2, cutoff value to define hyperoxia), prevalence of hyperoxia, overall in-hospital mortality, and prevalence of chronic cardiovascular and/or respiratory disease. Additional data were extracted for studies on post-cardiac arrest patients: average delay to return of spontaneous circulation (minutes); prevalence of initial shockable rhythm (ventricular tachycardia/fibrillation); prevalence of out-of-hospital cardiac arrest; prevalence of therapeutic hypothermia. Unadjusted outcome data (number of survivors and nonsurvivors to hospital discharge in hyperoxic and nonhyperoxic patients) and adjusted odds ratio (OR) (95% confidence interval) describing the association between hyperoxia exposure and mortality were extracted for calculation of effect size (ES). In-hospital mortality was chosen as the primary endpoint of our analysis since it was the outcome measure most frequently reported. When in-hospital mortality was not stated, we extracted data reporting the longest-term mortality available. The study authors were contacted to request additional information whenever a study did not report data necessary for calculation of the ES.
Study quality assessment
The Newcastle-Ottawa Scale (NOS) for cohort studies was used to assess the quality of the included studies [25]. The item ‘representativeness of the exposed cohort’ was fulfilled if ≤10% of patients had been excluded because of missing data. The item ‘completeness of follow-up’ was fulfilled if ≤10% of patients had been excluded because of missing mortality data. For assessment of comparability of cohorts, two confounding factors were defined a priori: illness severity (as defined by any of the following severity scores: Acute Physiology and Chronic Health Evaluation (APACHE), Simplified Acute Physiology Score (SAPS), Sequential Organ Failure Assessment (SOFA), Injury Severity Score (ISS)) and FiO2. A study was considered to adequately control for each of these factors if it either demonstrated balance between groups for the confounder, or adjusted for it in the statistical analysis.
Statistical analysis
Data were synthesized using meta-analytic methods [26],[27], and statistically pooled by the standard meta-analysis approach, that is studies were weighted by the inverse of the sampling variance. Whenever possible, calculation of the ES of individual studies was based on the adjusted OR of the association between hyperoxia exposure and mortality as compared to nonexposure. When the authors reported results from more than one multivariate model, we extracted data deriving from either the model that included the maximum number of covariates, or the model that included severity of illness and FiO2. If the authors analyzed the unadjusted or adjusted association between mortality and increasing quartiles/quintiles/deciles of PaO2, we considered patients as ‘hyperoxic’ if they fell in the upper stratum. When adjusted data were not reported or PaO2 was analyzed as a continuous variable, unadjusted ORs were reconstructed from binary raw data (number of survivors/nonsurvivors in hyperoxia exposed/not exposed). When normoxia and hypoxemia were considered as two separate categories, only normoxic patients were included in the comparator group. Overall ES was expressed as OR and its corresponding 95% confidence interval (CI). The DerSimonian and Laird random effects model was used as a conservative approach to account for different sources of variation among studies. Forest plots were constructed to graphically represent the results. Q statistics were used to assess heterogeneity among studies. A significant Q value indicates a lack of homogeneity of findings among studies [26],[27]. Inconsistency analysis (I 2) statistics were then used to quantify the proportion of observed inconsistency across study results not explained by chance [28]. I2 values of <25%, 50% and >75% represent low, moderate and high inconsistency, respectively [28]. Sensitivity analyses were planned a priori to assess the impact of potential outliers (based on statistical significance of the standardized residuals) and sources of heterogeneity. Several variables were identified and their effects on outcome examined. Categorical variables were treated as moderators and the strength of the association between hyperoxia and mortality assessed and compared across subgroups formed by these moderators. Continuous variables were examined as covariates using random effects meta-regression. Meta-regression was performed to assess the effect of study quality (NOS score) on the calculated estimate. The presence of publication bias was investigated through funnel plots both visually and formally by trim and fill analysis and Eggers’s linear regression method [29]. A P value less than 0.05 was used to indicate statistical significance. All analyses were conducted using a computer software package (ProMeta Version 2, Internovi, Cesena FC, Italy).
Results
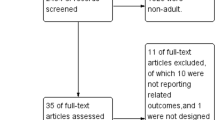
From the 2,389 articles that were initially identified (Figure 1), 70 potentially relevant original articles were examined in full text (κ = 0.87 (95% CI, 0.85 to 0.90)). Seventeen studies eventually met our inclusion criteria (κ = 0.91 (0.88 to 0.94)). The study by Kilgannon et al. [30] was excluded as it was a subgroup analysis of the same patient population previously described by the same group [17]. For the study by Ihle et al. [31] we extrapolated outcome data related to the years 2010 to 2011, as the authors used the same database as Bellomo et al. [21] with study populations overlapping for the years 2007 to 2009. Two studies were identified in which hyperoxia exposure was defined on the basis of a peripheral O2 saturation (SpO2) >98% [2],[32]; although it is questionable whether these patients were really hyperoxic to a significant degree, we decided to include these studies in the analysis as the reported time-weighted PaO2 values were above the upper normal limit of 100 mmHg in both cases [2],[32].
The 17 studies identified were all published in English between 2008 and 2014 and involved different categories of critically ill patients [2],[16]-[23],[31]-[38]. Only four studies (24%) involved general populations of mechanically ventilated intensive care unit (ICU) patients [2],[16],[20],[32]. Six studies focused upon patients resuscitated from cardiac arrest [17],[21],[31],[35]-[37], five studies evaluated patients with traumatic brain injury [18],[22],[33],[34],[38], while two studies involved patients with stroke [19],[23]. The main characteristics of the studies are reported in Table 1. Individual unadjusted/adjusted outcome data and variables included in the multivariable models are reported in Table 2. Study quality assessment is reported in Additional file 2.
Mechanically ventilated ICU patients
The four studies including general populations of mechanically ventilated ICU patients (number of participants (n) = 189,143) were highly heterogeneous in terms of design, outcome measure, criteria for defining hyperoxia exposure, and statistical method applied for analysis (Table 1). de Jonge et al. [16] defined the worst PaO2 as the one associated with the lowest PaO2/FiO2. Conversely, Eastwood et al. [20] defined the worst PaO2 in patients with an FiO2 ≥0.5 as that associated with the ABG providing the highest arterial-alveolar (A-a) gradient; for patients with an FiO2 <0.5, the lowest PaO2 was recorded. If arterial blood gases (ABGs) were taken in patients in whom FiO2 <0.5 and ≥0.5 were both recorded during the first 24 hours, the value of PaO2 taken when the FiO2 ≥0.5 was used. In one study [2] hyperoxia was defined on the basis of a time-weighted SpO2 >98%; this parameter was calculated as the mean SpO2 between consecutive time points multiplied by the period of time between these time points, with the sum of such time-weighted values being divided by total time to obtain a time-weighted average. For the before-after study by Suzuki et al. [32], we considered the ‘conservative’ group as not exposed to arterial hyperoxia and the ‘conventional’ group as exposed to arterial hyperoxia (showing a time-weighted average SpO2 of 98.4%).
Extreme heterogeneity was found among the study findings (Q (3) = 91.85, P <0.001; I 2 = 96.73), with an ES ranging from 0.73 to 2.86. Individual ES values (95% CI) are shown in Figure 2. A pooled estimate was not calculated in view of the insufficient homogeneity.
Forest plot showing individual odds ratios for mortality of studies on general populations of mechanically ventilated ICU patients (k = 4). Odds ratios >1 (right side of the plot) indicate an association between hyperoxia and higher mortality. Heterogeneity was Q (3) 91.85, P <0.001; I 2 = 96.73. The size of the boxes is inversely proportional to the size of the result study variance, so that more precise studies have larger boxes. k, number of studies; ES, effect size; CI, confidence interval; Sig., P value.
Patients resuscitated from cardiac arrest
Six retrospective cohort studies (three multicenter [17],[21],[31], three single-center [35]-[37]) evaluated patients resuscitated from cardiac arrest (n = 19,144). Different PaO2 measures were used to define hyperoxia exposure (Table 1). Most studies used a PaO2 cutoff of 300 mmHg [17],[21],[31],[37]. In the study by Janz et al. [35], the relationship between mortality and PaO2 as a continuous variable was evaluated by multivariable regression analysis; to limit heterogeneity in both hyperoxia definition and analysis between this and the other studies, we reconstructed the unadjusted binary OR by stratifying patients between hyperoxic and nonhyperoxic groups based on a 300 mmHg PaO2 cutoff and extracting the number of survivors/nonsurvivors in the two groups. The study by Lee et al. [36] was not included in the quantitative synthesis because of the substantial differences found in comparison to the other reported studies (different criteria for defining hyperoxia).
The pooled ES shows an association between hyperoxia exposure and increased in-hospital mortality (OR = 1.42 (95% CI 1.04 to 1.92), P = 0.028, number of studies (k) = 5), in the presence of a moderately high heterogeneity (Q (4) = 12.4, P = 0.015; I 2 = 67.73) (Figure 3). A funnel plot indicates no obvious publication bias (Additional file 3).
Forest plot showing individual and pooled odds ratios for mortality of studies on patients resuscitated from cardiac arrest. Odds ratios >1 (right side of the plot) indicate an association between hyperoxia and higher mortality. Heterogeneity was Q (4) = 12.4, P = 0.015; I 2 = 67.73. The size of the boxes is inversely proportional to the size of the result study variance; more precise studies have larger boxes. ES, effect size; CI, confidence interval; W, weight; Sig., P value.
Results of the moderator analyses are shown in Table 3. A significant overall ES was found among multicenter (k = 3) but not single-center (k = 2) studies. A significant association with mortality was indicated only in the study that used the first available PaO2. An association of borderline statistical significance was shown only for studies in which adjusted data were used. Meta-regression analyses showed a progressively weaker association with increasing prevalence of chronic cardiovascular disease (k = 4, range 12 to 36%). No significant moderator effect was shown by the following variables: mean age (k = 5; range 60.5 to 64 years); gender (k = 4; range 54 to 80% of males); hospital mortality (k = 5; range 32.4 to 66%); average delay to return of spontaneous circulation (k = 3; range 15 to 25.7 minutes); prevalence of initial shockable rhythm (ventricular tachycardia/fibrillation, k = 3; range 40 to 100%); prevalence of out-of-hospital cardiac arrest (k = 5; range 43 to 100%). Study quality as defined by the NOS score had no effect on the ES.
Patients with stroke
Two multicenter retrospective cohort studies [19],[23] evaluated the relationship between in-hospital mortality and exposure to arterial hyperoxia in the first 24 hours of ICU admission in patients with stroke (n = 5,537). Rincon et al. [19] defined patients with the first PaO2 ≥300 mmHg as being exposed to hyperoxia. Young et al. [23] evaluated the independent association between mortality and deciles of PaO2, with the upper decile (PaO2 >341 mmHg) used as the reference category. To make the two studies comparable, for [23] we considered patients in the upper decile as those being exposed to hyperoxia and reconstructed the unadjusted OR for mortality; patients in the first decile (PaO2 ≤69 mmHg) were excluded. The pooled ES indicates an association between hyperoxia exposure and increased hospital mortality (OR = 1.23 (95% CI 1.06 to 1.43), P = 0.005; Q (1) = 0.04, P = 0.844, I 2 = 0) (Figure 4).
Forest plot showing individual and pooled odds ratios for mortality of studies on patients with stroke. Odds ratios >1 (right side of the plot) indicate an association between hyperoxia and higher mortality. Heterogeneity was Q (1) = 0.04, P = 0.844, I 2 = 0. The size of the boxes is inversely proportional to the size of the result study variance, so that more precise studies have larger boxes. ES, effect size; CI, confidence interval; W, weight; Sig., P value.
Patients with traumatic brain injury
Four multicenter [18],[22],[34],[38] and one single-center [33] retrospective cohort studies evaluated patients with traumatic brain injury (n = 7,488). Different criteria were used to define hyperoxia in terms of time of assessment, PaO2 selection and cutoff value used (Table 1). All studies reported the adjusted ORs for hospital mortality (Table 2).
The pooled ES indicates an association between hyperoxia exposure and increased mortality (OR = 1.41 (95% CI 1.03 to 1.94), P = 0.032) in the presence of significant heterogeneity (Q (4) = 11.28, P = 0.024; I 2 = 64.54) (Figure 5). The funnel plot indicated no obvious publication bias (Additional file 4). The exclusion of the study by Asher et al. [33] (single-center, time of PaO2 assessment beyond the first 24 hours) in the sensitivity analysis did not substantially change the combined ES (OR = 1.46 (95% CI 1.08 to 1.98)) nor did it decrease heterogeneity (I 2 = 67.29). Results of the moderator analyses are shown in Table 4, with studies stratified based on design, PaO2 value and cutoff used for defining hyperoxia, time of PaO2 assessment and comparator group. Study quality as defined by the NOS score, mean age and gender did not influence the ES.
Forest plot showing individual and pooled odds ratio for mortality of studies on patients with traumatic brain injury. Odds ratios >1 (right side of the plot) indicate an association between hyperoxia and higher mortality. Heterogeneity was Q (4) = 11.28, P = 0.024; I 2 = 64.54. The size of the boxes is inversely proportional to the size of the result study variance; more precise studies have larger boxes. ES, effect size; CI, confidence interval; W, weight; Sig., P value.
Discussion
The main findings of our systematic review and meta-analysis may be summarized in three points. First, the majority of studies that explored the relationship between arterial hyperoxia and mortality were retrospective observational investigations, with only one interventional prospective before-after study comparing a conventional ventilation strategy against a more conservative strategy. RCTs comparing two different PaO2/SpO2-targeted ventilation strategies are lacking. Second, high heterogeneity was found between studies in the criteria used for defining hyperoxia exposure (PaO2 value and cutoff selected, time of assessment) and the statistical method applied for analysis; this limits comparison between the study results. Third, while studies in general populations of ICU patients gave highly inconsistent results, an association between arterial hyperoxia and increased hospital mortality was found in critically ill patient subsets (post-cardiac arrest, stroke, traumatic brain injury).
Four studies evaluated general populations of mechanically ventilated patients [2],[16],[20],[32] and gave highly inconsistent results. Two large multicenter retrospective studies [16],[20] reported a U-shaped relationship between PaO2 levels and mortality by unadjusted analysis. After adjusting for potential confounders including severity of illness, the association between higher PaO2 levels and mortality was confirmed only by de Jonge et al. [16], while Eastwood et al. [20] showed a protective effect of hyperoxia. Differences in the methods applied for the analysis (PaO2 stratified in quintiles/deciles, different reference categories) make these studies difficult to compare. A pilot before-after trial was the only interventional study that compared conventional management to a conservative strategy using an SpO2 target between 90 and 92% [32]. Although this study was underpowered to demonstrate a difference in mortality, it supported the feasibility and safety of a restrictive O2 therapy, which led to a marked reduction in O2 exposure without being associated with major clinical and physiological adverse effects.
Our analysis showed a significant association between hyperoxia exposure and mortality in patients resuscitated from cardiac arrest. This is consistent with the result of a recent meta-analysis by Wang et al. [39]. In a meta-analysis of animal trials, the administration of 100% O2 therapy following resuscitation from cardiac arrest was associated with worse neurological outcomes as compared with lower O2 concentrations [40]. An association between hyperoxia exposure and poor neurological outcomes has been reported by several authors [17],[35],[36],[41], but not confirmed by a recent multicenter observational study [42] that instead highlighted PaCO2 as a possible confounding factor. The adverse effects of hyperoxia may be due to enhanced oxidative stress, which may be particularly deleterious during the early reperfusion phase after cardiac arrest, and a vasoconstrictor effect that may paradoxically lead to a net reduction in local O2 delivery to tissues including the myocardium and brain [43]. Mechanisms by which hyperoxia causes vasoconstriction include an inhibition of vasodilator (prostaglandins, nitric oxide) by reactive O2 species [43]. Of note, the strength of association between hyperoxia and mortality was inversely related to the prevalence of chronic cardiovascular disease. We speculate that the response to a high O2 tension may be blunted in the presence of an underlying endothelial dysfunction, where nitric oxide levels may be chronically low [44]. However, this hypothesis can be challenged by data showing a deleterious effect of high O2 in patients with severe coronary artery disease and myocardial infarction [45],[46]. An alternative hypothesis may be that hyperoxia exposure had a minor role on mortality when the prevalence of cardiovascular comorbidity was higher.
The exposure to arterial hyperoxia in the first 24 hours of ICU admission was associated with higher mortality in patients with stroke, although this result is limited by the low number of studies included. Previous RCTs showed only transient radiological (magnetic resonance imaging) and clinical improvement [47], no benefit [48] or worse outcomes [49] in stroke patients receiving supplemental O2 during their initial management. The Stroke Oxygen Study RCT, due to report in early 2016, is comparing three-day continuous or nocturnal O2 administration with no supplemental O2 in 6,000 patients (ISRCTN52416964, http://www.controlled-trials.com). Likewise, arterial hyperoxia was associated with increased hospital mortality in patients with traumatic brain injury. This should, however, be interpreted with caution given the heterogeneous characteristics of the studies included. The rationale for giving high O2 concentrations to patients with traumatic brain injury is to improve brain O2 delivery and metabolism [50]. While studies using indirect measures of brain metabolism provided promising results [7], these were not subsequently confirmed by a study that found no improvement in brain O2 utilization measured by positron emission tomography one hour after ventilation with 100% O2 [51].
The different criteria used for defining hyperoxia exposure were the main source of heterogeneity among the analyzed studies. The selection of ‘worst’ PaO2 based on the A-a gradient [20]-[23],[31] has been questioned by several authors, as this gradient does not correlate in a linear fashion with PaO2 as the FiO2 increases [35] and this method may reduce the probability of finding any association between hyperoxia and mortality [52]. However, a subanalysis by Bellomo et al. [21] on a sample of 100 patients showed that the worst PaO2 was more representative of mean PaO2 than was the first PaO2 measured after ICU admission. Different PaO2 measures may have different pathophysiological consequences. If even short periods of hyperoxia were dangerous, then the highest PaO2 would represent the most sensitive approach to identify patients at risk; conversely, if the overall effect depended on the total amount of excess O2 received, then the mean PaO2 or a time-weighted measure would be a better choice. Alternatively, the first PaO2 measurement would be preferable if the deleterious effects of hyperoxia were more pronounced in the early phase of the disease. Regardless of disease category, all studies that considered the first available PaO2 [17],[19],[38] found an independent association between hyperoxia exposure and hospital mortality, while studies using other measures showed more inconsistent results. This may suggest that exposure to high O2 tensions in the early phase of the critical illness may be particularly associated with worse outcomes. Interestingly, in a subgroup analysis by de Jonge et al. [16], exposure to hyperoxia as defined by higher mean PaO2 values during the entire ICU stay was not independently associated with mortality. All studies that considered a timespan longer than the first 24 hours for assessing oxygenation status did not find any significant association between arterial hyperoxia and worse outcomes [2],[33],[36].
In most of the studies, patients were categorized as hyperoxic or nonhyperoxic based on an arbitrarily predetermined PaO2 cutoff value. Similarly, our analysis was based on binary ORs of hyperoxia exposure versus nonexposure. This approach may be limited by poor resolution in describing the relationship between increasing arterial O2 tensions and outcome. Several studies in which PaO2 was analyzed as a continuous variable showed a linear relationship between increasing arterial O2 tensions and mortality, without a clear threshold for harm [30],[35]. Furthermore, there is no consensus on the PaO2 cutoff value to use for defining hyperoxia exposure, which varied markedly across the analyzed studies. This is likely to influence the associations observed. In a meta-regression analysis on the overall set of studies, the association between hyperoxia and worse outcome appeared to become stronger when the PaO2 cutoff value used for defining exposure increased (data shown in Additional file 5).
Strengths and weaknesses
This is the first systematic review on the relationship between arterial hyperoxia and mortality in critically ill patients that gathers together data from a large number of subjects within several distinct disease categories. In our quantitative data syntheses, every effort was made to control for possible sources of heterogeneity and confounding factors. The authors were contacted if additional unpublished data were needed; any overlap between study populations was avoided. Whenever possible, adjusted outcome data were used and/or hypoxic patients excluded. Moderator analyses were performed to analyze the impact of several sources of heterogeneity (definition of hyperoxia exposure, study design). Study quality was assessed by means of a standardized scale and its impact on the studied association was explored. A random-effects model was used to pool data to account for unmeasured confounding factors and sources of heterogeneity.
Our analysis has several limitations. First, the studies were mainly observational investigations that cannot directly support any causal relationship between hyperoxia exposure and worse outcome. Higher PaO2 levels may simply reflect the clinicians’ attempts to optimize O2 delivery by administering a higher FiO2; thus PaO2 becomes a marker of illness severity rather than being directly responsible for the outcome. Second, the included studies used different criteria for defining hyperoxia exposure and applied different statistical methods for analysis (PaO2 as an ordinal/continuous variable; different multivariable regression models). Third, hypoxemic patients could not always be excluded from the analysis: these patients are likely to be responsible for an increased mortality in the subgroup of those not exposed to hyperoxia and might thus have blunted the studied association. Finally, we did not include unpublished studies, dissertations, or conference abstracts. We decided to consider only published material to ensure that only higher quality, peer-reviewed studies were included in the analysis.
Clinical implications and directions for future research
Given the widespread use of O2 therapy in critical care, clinicians should be aware of the potentially deleterious effects of excessive O2 administration. Several studies reported that FiO2 is rarely adjusted for arterial hyperoxia, especially when this occurs at lower FiO2 settings [1],[2]. The rationale for giving supplemental O2 to nonhypoxemic patients should be reconsidered as there is insufficient evidence of benefit [43]. When hemoglobin is fully saturated, additional O2 only marginally increases O2 transport capacity; conversely, a paradoxical decrease in regional O2 delivery could be caused by vasoconstriction [53].
An urgent need exists for adequately designed studies to provide conclusive answers regarding the safety of hyperoxia in critically ill patients. Only RCTs can confirm a causal relationship between hyperoxia exposure and higher mortality. These trials should evaluate ventilation strategies using different PaO2 targets for titrating FiO2, rather than comparing two arbitrarily selected FiO2 targets. A pilot before-after study has supported the safety and feasibility of a conservative oxygen therapy [32]. RCTs comparing current liberal ventilation practices to more restrictive approaches in ICU patients are currently ongoing (ClinicalTrials.gov, NCT01319643 and NCT01722422).
There is an imperative to identify the best criteria that define hyperoxia exposure in observational studies. The relationship between hyperoxia and mortality should be evaluated using different criteria for defining hyperoxia exposure, comparing different PaO2 measures and the time of assessment. This analysis may have important pathophysiological implications and could clarify whether early exposure to hyperoxia during critical illness is more deleterious. In future research, it would also be more useful to assess and report the relationship between mortality and PaO2 as a continuous variable, instead of stratifying patients on the basis of an arbitrary PaO2 cutoff value. Finally, further studies should address other specific categories of critically ill patients, such as sepsis, polytrauma, post-operative cases and hemorrhagic shock.
Conclusions
The majority of studies that have explored the relationship between arterial hyperoxia and mortality in critically ill patients are retrospective observational investigations, with only one prospective before-after study supporting the safety of a more conservative strategy. A quantitative data synthesis was not possible for studies on general populations of mechanically ventilated ICU patients because of differences in design, definition of hyperoxia, and the outcome measure reported. Hyperoxia exposure may be associated with mortality in patient subsets (post-cardiac arrest, stroke and traumatic brain injury). However, these results must be interpreted cautiously given the heterogeneity in criteria used for defining hyperoxia exposure and a significant inconsistency between study findings. Nevertheless, these data provide the rationale for future RCTs comparing conventional practice against more restrictive oxygenation targets.
Key messages
-
There is insufficient evidence regarding the safety of arterial hyperoxia in critically ill patients. Most of the existing studies are observational investigations with highly heterogeneous characteristics and inconsistent results. Randomized controlled trials are lacking.
-
Arterial hyperoxia may be associated with higher mortality in some critically ill patient subsets (post-cardiac arrest, stroke and traumatic brain injury).
Additional files
Abbreviations
- (A-a):
-
alveolar-arterial
- ABG:
-
arterial blood gas
- ALI:
-
acute lung injury
- APACHE:
-
Acute Physiology and Chronic Health Evaluation
- CI:
-
confidence interval
- COPD:
-
chronic obstructive pulmonary disease
- ES:
-
effect size
- FiO2:
-
inspired oxygen fraction
- I2:
-
inconsistency analysis
- ICU:
-
intensive care unit
- ISS:
-
Injury Severity Score
- k:
-
number of studies
- n:
-
number of participants
- NOS:
-
Newcastle-Ottawa Scale
- O2:
-
oxygen
- OR:
-
odds ratio
- PaO2:
-
arterial partial oxygen pressure
- RCT:
-
randomized controlled trial
- SAPS:
-
Simplified Acute Physiology Score
- SOFA:
-
Sequential Organ Failure Assessment
- SpO2:
-
peripheral oxygen saturation
References
de Graaf AE, Dongelmans DA, Binnekade JM, de Jonge E: Clinicians’ response to hyperoxia in ventilated patients in a Dutch ICU depends on the level of FiO2. Intensive Care Med. 2011, 37: 46-51. 10.1007/s00134-010-2025-z.
Suzuki S, Eastwood GM, Peck L, Glassford NJ, Bellomo R: Current oxygen management in mechanically ventilated patients: a prospective observational cohort study. J Crit Care. 2013, 28: 647-654. 10.1016/j.jcrc.2013.03.010.
Task Force for Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of European Society of Cardiology, Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Strömberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K, ESC Committee for Practice Guidelines, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, et al: ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the task force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the heart failure association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008, 29: 2388-2442. 10.1093/eurheartj/ehn309.
Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr,Chavey WE II, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED,Theroux P, Wenger NK, Wright RS, Smith SC Jr, Jacobs AK, Adams CD,Damiani et al. Critical Care (2014) 18:711 Page 14 of 16Anderson JL, Antman EM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG,Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B, American College ofCardiology, American Heart Association Task Force on Practice Guidelines(Writing Committee to Revise the 2002 Guidelines for the Management ofPatients With Unstable Angina/Non-ST-Elevation Myocardial Infarction),American College of Emergency Physicians, Society for CardiovascularAngiography and Interventions, Society of Thoracic Surgeons, AmericanAssociation of Cardiovascular and Pulmonary Rehabilitation, Society forAcademic Emergency Medicine: ACC/AHA 2007 guidelines for themanagement of patients with unstable angina/non-ST-elevationmyocardial infarction: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (writingcommittee to revise the 2002 guidelines for the management ofpatients with unstable angina/non-ST-elevation myocardial infarction)developed in collaboration with the American College of EmergencyPhysicians, the Society for Cardiovascular Angiography and Interventions,and the Society of Thoracic Surgeons endorsed by the AmericanAssociation of Cardiovascular and Pulmonary Rehabilitation and the Societyfor Academic Emergency Medicine. J Am Coll Cardiol 2007, 50:e1–e157.
O’Driscoll R, Davison A, Elliott M, Howard L, Wedzicha J, Mackway-Jones K, Jenkins P, Kishen R, Levy M, Perrott S, Mansfield L, Evans A, Panizzo S, Moore F, Whitmore D, Gibbs S, Martin B, Hinshaw K: BTS guideline for emergency oxygen use in adult patients. Thorax. 2008, 63: vi1-68-
Peberdy MA, Callaway CW, Neumar RW, Geocadin RG, Zimmerman JL, Donnino M, Gabrielli A, Silvers SM, Zaritsky AL, Merchant R, Vanden Hoek TL, Kronick SL: Part 9: post-cardiac arrest care: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010, 2010: 768-786. 10.1161/CIRCULATIONAHA.110.971002.
Kumaria A, Tolias CM: Normobaric hyperoxia therapy for traumatic brain injury and stroke: a review. Br J Neurosurgery. 2009, 23: 576-584. 10.3109/02688690903050352.
Menzel M, Doppenberg EM, Zauner A, Soukup J, Reinert MM, Bullock R: Increased inspired oxygen concentration as a factor in improved brain tissue oxygenation and tissue lactate levels after severe human head injury. J Neurosurg. 1999, 91: 1-10. 10.3171/jns.1999.91.1.0001.
Singhal AB: Oxygen therapy in stroke: past, present, and future. Int J Stroke. 2006, 1: 191-200. 10.1111/j.1747-4949.2006.00058.x.
Fracica PJ, Knapp MJ, Piantadosi CA, Takeda K, Fulkerson WJ, Coleman RE, Wolfe WG, Crapo JD: Responses of baboons to prolonged hyperoxia: physiology and qualitative pathology. J Appl Physiol. 1991, 71: 2352-2362.
Crapo JD, Hayatdavoudi G, Knapp MJ, Fracica PJ, Wolfe WG, Piantadosi CA: Progressive alveolar septal injury in primates exposed to 60% oxygen for 14 days. Am J Physiol. 1994, 267: L797-L806.
Altemeier WA, Sinclair SE: Hyperoxia in the intensive care unit: why more is not always better. Curr Opin Crit Care. 2007, 13: 73-78. 10.1097/MCC.0b013e32801162cb.
Lodato RF: Decreased O2 consumption and cardiac output during normobaric hyperoxia in conscious dogs. J Appl Physiol. 1989, 67: 1551-1559.
Chan PH: Reactive oxygen radicals in signaling and damage in the ischaemic brain. J Cereb Blood Flow Metab. 2001, 21: 2-14. 10.1097/00004647-200101000-00002.
Farquhar H, Weatherall M, Wijesinghe M, Perrin K, Ranchord A, Simmonds M, Beasley R: Systematic review of studies of the effect of hyperoxia on coronary blood flow. Am Heart J. 2009, 158: 371-377. 10.1016/j.ahj.2009.05.037.
de Jonge E, Peelen L, Keijzers PJ, Joore H, de Lange D, van der Voort PHJ, Bosman RJ, de Waal RAL, Wesselink R, de Keizer NF: Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care. 2008, 12: R156-10.1186/cc7150.
Kilgannon JH, Jones AE, Shapiro NI, Angelos MG, Milcarek B, Hunter K, Parrillo JE, Trzeciak S for the Emergency Medicine Shock Research Network (EMShockNet) Investigators: Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010, 303: 2165-2171. 10.1001/jama.2010.707.
Brenner M, Stein D, Hu P, Kufera J, Wooford M, Scalea T: Association between early hyperoxia and worse outcome after traumatic brain injury. Arch Surg. 2012, 147: 1042-1046. 10.1001/archsurg.2012.1560.
Rincon F, Kang J, Maltenfort M, Vibbert M, Urtecho J, Athar MK, Jallo J, Pineda CC, Tzeng D, McBride W, Bell R: Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit Care Med. 2014, 42: 387-396. 10.1097/CCM.0b013e3182a27732.
Eastwood G, Bellomo R, Bailey M, Taori G, Pilcher D, Young P, Beasley R: Arterial oxygen tension and mortality in mechanically ventilated patients. Intensive Care Med. 2012, 38: 91-98. 10.1007/s00134-011-2419-6.
Bellomo R, Bailey M, Eastwood GM, Nichol A, Pilcher D, Hart GK, Reade MC, Egi M, Cooper DJ, the Study of Oxygen in Critical Care (SOCC) group: Arterial hyperoxia and in-hospital mortality after resuscitation from cardiac arrest. Crit Care. 2011, 15: R90-10.1186/cc10090.
Raj R, Bendel S, Reinikainen M, Kivisaari R, Siironen J, Lang M, Skrifvars M: Hyperoxemia and long-term outcome after traumatic brain injury. Crit Care. 2013, 17: R177-10.1186/cc12856.
Young P, Beasly R, Baily M, Bellomo R, Eastwood GM, Nichol A, Pilcher DV, Yunos MN, Egi M, Hart GK, Reade MC, Cooper DJ, for the Study of Oxygen in Critical Care (SOCC) group: The association between early arterial oxygenation and mortality in ventilated patients with acute ischemic stroke. Crit Care Res. 2012, 14: 14-19.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group: Preferred Reporting Items for Systematic Reviews and Meta-Analysis: the PRISMA statement. PLOS Med. 2009, 6: e1000097-10.1371/journal.pmed.1000097.
Downs SH, Black N: The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998, 52: 377-384. 10.1136/jech.52.6.377.
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR: Introduction to Meta-Analysis. 2009, Chichester, UK, John Wiley and Sons, Ltd
Practical Meta-Analysis. 2001, Sage, Thousand Oaks, CA
Higgins JPT, Thompson SG, Deeks JJ, Altman DG: Measuring inconsistency in meta-analysis. BMJ. 2003, 327: 557-560. 10.1136/bmj.327.7414.557.
Sterne JAC, Egger M: Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001, 54: 1046-1055. 10.1016/S0895-4356(01)00377-8.
Kilgannon JH, Jones AE, Parrillo JE, Dellinger RP, Milcarek B, Hunter K, Shapiro NI, Trzeciak S, on behalf of the Emergency Medicine Shock Research Network (EMShockNet) Investigators: Relationship between supranormal oxygen tension and outcome after resuscitation from cardiac arrest. Circulation. 2011, 123: 2717-2722. 10.1161/CIRCULATIONAHA.110.001016.
Ihle JF, Bernard S, Bailey MJ, Pilcher DV, Smith K, Scheinkestel CD: Hyperoxia in the intensive care unit and outcome after out-of-hospital ventricular fibrillation cardiac arrest. Crit Care Resusc. 2013, 15: 186-190.
Suzuki S, Eastwood GM, Glassford NJ, Peck L, Young H, Garcia-Alvarez M, Schneider AG, Bellomo R: Conservative oxygen therapy in mechanically ventilated patients: a pilot before-and-after trial. Crit Care Med. 2014, 42: 1414-1422. 10.1097/CCM.0000000000000219.
Asher SR, Curry P, Sharma D, Wang J, O’Keefe GE, Daniel-Johnson J, Vavilala MS: Survival advantage and PaO2 threshold in severe traumatic brain injury. J Neurosurg Anesthesiol. 2013, 25: 168-173. 10.1097/ANA.0b013e318283d350.
Davis DP, Meade W, Sise MJ, Kennedy F, Simon F, Tominaga G, Steele J, Coimbra R: Both hypoxemia and extreme hyperoxemia may be detrimental in patients with severe traumatic brain injury. J Neurotrauma. 2009, 26: 2217-2223. 10.1089/neu.2009.0940.
Janz DR, Hollenbeck RD, Pollock JS, McPherson JA, Rice TW: Hyperoxia is associated with increased mortality in patients treated with mild therapeutic hypothermia after sudden cardiac arrest. Crit Care Med. 2012, 40: 3135-3139. 10.1097/CCM.0b013e3182656976.
Lee BK, Jeung KW, Lee HY, Lee SJ, Jung YH, Lee WK, Heo T, Min YI: Association between mean arterial blood gas tension and outcome in cardiac arrest patients treated with therapeutic hypothermia. Am J Emerg Med. 2014, 32: 55-60. 10.1016/j.ajem.2013.09.044.
Nelskyla A, Parr MJ, Skrifvars MB: Prevalence and factors correlating with hyperoxia exposure following cardiac arrest – an observational single centre study. Scan J Trauma Resusc Emerg Med. 2013, 21: 35-10.1186/1757-7241-21-35.
Rincon F, Kang J, Vibbert M, Urtecho J, Athar MK, Jallo J: Significance of arterial hyperoxia and relationship with case fatality in traumatic brain injury: a multicentre cohort study. J Neurol Neurosurg Psychiatry. 2014, 85: 799-805. 10.1136/jnnp-2013-305505.
Wang CH, Chang WT, Huang CH, Tsai MS, Yu PH, Wang AY, Chen NC, Chen WJ: The effect of hyperoxia on survival following adult cardiac arrest: a systematic review and meta-analysis of observational studies. Resuscitation. 2014, 85: 1142-1148. 10.1016/j.resuscitation.2014.05.021.
Pilcher J, Weatherall M, Shirtcliffe P, Bellomo R, Young P, Beasley R: The effect of hyperoxia following cardiac arrest – A systematic review and meta-analysis of animal trials. Resuscitation. 2012, 83: 417-422. 10.1016/j.resuscitation.2011.12.021.
Kuisma M, Boyd J, Voipio V, Alaspää A, Roine RO, Rosenberg P: Comparison of 30 and the 100% inspired oxygen concentrations during early post-resuscitation period: a randomized controlled pilot study. Resuscitation. 2006, 69: 199-206. 10.1016/j.resuscitation.2005.08.010.
Vaahersalo J, Bendel S, Reinikainen M, Kurola J, Tiainen M, Raj R, Pettila V, Varpula T, Skrifvars MB, for the FINNRESUSCI Study Group: Arterial blood gas tensions after resuscitation from out-of-hospital cardiac arrest: associations with long-term neurological outcome. Crit Care Med. 2014, 42: 1463-1470. 10.1097/CCM.0000000000000228.
Iscoe S, Beasley R, Fisher JA: Supplementary oxygen for non-hypoxemic patients: O2 much of a good thing?. Crit Care. 2011, 15: 305-10.1186/cc10229.
Li H, Forstermann U: Uncoupling of endothelial NO synthase in atherosclerosis and vascular disease. Curr Opin Pharmacol. 2013, 13: 161-167. 10.1016/j.coph.2013.01.006.
Bourassa MG, Campeau L, Bois MA, Rico O: The effects of inhalation of 100 percent oxygen on myocardial lactate metabolism in coronary heart disease. Am J Cardiol. 1969, 24: 172-177. 10.1016/0002-9149(69)90400-7.
Rawles JM, Kenmure AC: Controlled trial of oxygen in uncomplicated myocardial infarction. BMJ. 1976, 1: 1121-1123. 10.1136/bmj.1.6018.1121.
Singhal AB, Benner T, Roccatagliata L, Koroshetz WJ, Schaefer PW, Lo EH, Buonanno FS, Gonzalez RG, Sorensen AG: A pilot study of normobaric oxygen therapy in acute ischemic stroke. Stroke. 2005, 36: 797-802. 10.1161/01.STR.0000158914.66827.2e.
Padma MV, Bhasin A, Bhatia R, Garg A, Singh MB, Tripathi M, Prasad K: Normobaric oxygen therapy in acute ischemic stroke: a pilot study in Indian patients. Ann Indian Acad Neurol. 2010, 13: 284-288. 10.4103/0972-2327.74203.
Ronning OM, Guldvog B: Should stroke victims routinely receive supplemental oxygen? A quasi-randomized controlled trial. Stroke. 1999, 30: 2033-2037. 10.1161/01.STR.30.10.2033.
Diringer MN: Hyperoxia – good or bad for the injured brain?. Curr Opin Crit Care. 2008, 14: 167-171. 10.1097/MCC.0b013e3282f57552.
Diringer MN, Aiyagari V, Zazulia AR, Videen TO, Powers WJ: Effect of hyperoxia on cerebral metabolic rate for oxygen measured using positron emission tomography in patients with acute severe head injury. J Neurosurg. 2007, 106: 526-529. 10.3171/jns.2007.106.4.526.
O’Driscoll BR, Howard LS: How to assess the dangers of hyperoxemia: methodological issues. Crit Care. 2011, 15: 435-10.1186/cc10272.
Cornet AD, Kooter AJ, Peters MJL, Smulders YM: The potential harm of oxygen therapy in medical emergencies. Crit Care. 2013, 17: 313-10.1186/cc12554.
Acknowledgements
We thank the authors Dr JF Ihle, Dr DR Janz, Dr BK Lee and Dr MS Vavilala for kindly answering our queries and providing additional information regarding their studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ED contributed to the study conception and design, acquisition, analysis and interpretation of data, and drafting of the manuscript. EA and AD contributed to the study conception, data acquisition, interpretation of results, and drafting of the manuscript. MG, RR, PP and MS made substantial contributions to both study design and data interpretation plus critical revision of the manuscript. All authors have read and approved the final version of the manuscript. All authors agree to be accountable for all aspects of the work ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Electronic supplementary material
13054_2014_711_MOESM1_ESM.pdf
Additional file 1: Search strategy. Search strategy applied for Medline (PubMed) and adapted for the other electronic databases. (PDF 179 KB)
13054_2014_711_MOESM2_ESM.pdf
Additional file 2: Study quality assessment with the Newcastle-Ottawa Scale (NOS). Items fulfilled are indicated by an ‘*’. (PDF 287 KB)
13054_2014_711_MOESM3_ESM.tiff
Additional file 3: Funnel plot related to the five studies evaluating patients resuscitated from cardiac arrest. Funnel plot analysis did not show any asymmetry. (TIFF 571 KB)
13054_2014_711_MOESM4_ESM.tiff
Additional file 4: Funnel plot related to the five studies evaluating patients with traumatic brain injury. Funnel plot analysis did not show any asymmetry. (TIFF 557 KB)
13054_2014_711_MOESM5_ESM.tiff
Additional file 5: Meta-regression analysis showing the impact on the study ES of the PaO 2 cutoff used for defining hyperoxia. Each circle represents a study. The size of the circles is inversely proportional to the size of the result study variance, so that more precise studies have larger circles. Meta-regression analysis showed that the strength of the association between arterial hyperoxia and mortality increased with increasing PaO2 cutoff values used for defining hyperoxia exposure. (TIFF 1 MB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Damiani, E., Adrario, E., Girardis, M. et al. Arterial hyperoxia and mortality in critically ill patients: a systematic review and meta-analysis. Crit Care 18, 711 (2014). https://doi.org/10.1186/s13054-014-0711-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-014-0711-x