Abstract
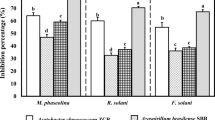
The aim of the present work was to test known bacterial plant growth-promoting strains for their ability to promote cucumber plant growth in salinated soil and to improve cucumber fruit yield by protecting these plants against soil-borne pathogens. Fifty-two plant-beneficial bacterial strains were evaluated for their ability to protect plants against cucumber foot and root rot after bacterization of the seeds and infestation of salinated soil with the isolated Fusarium solani pathogen. Based on the results of initial screenings, five efficient strains were selected, namely Serratia plymuthica RR-2-5-10, Stenotrophomonas rhizophila e-p10, Pseudomonas fluorescens SPB2145, Pseudomonas extremorientalis TSAU20, and P. fluorescens PCL1751. All five strains are salt tolerant since they grow well in a medium to which 3% NaCl was added. Infestation of the soil with F. solani resulted in an increase of the percentage of diseased plants from 17 to 54. Priming of seedlings with the five selected bacterial strains reduced this proportion to as low as 10%. In addition, in the absence of an added pathogen, all five strains showed a significant stimulatory effect on cucumber plant growth, increasing the dry weight of whole cucumber plants up to 62% in comparison to the non-bacterized control. The strains also increased cucumber fruit yield in greenhouse varying from 9% to 32%. We conclude that seed priming with the selected microbes is a very promising approach for improving horticulture in salinated soils. Moreover, allochthonous strains isolated from non-salinated soil, from a moderate or even cold climate, and from other plants than cucumber, functioned as well as autochthonous strains as cucumber-beneficial bacteria in salinated Uzbek soils. These results show that these plant-beneficial strains are robust and they strongly suggest they can also be used successfully in case the climate gets warmer and the soils will become more salinated. Finally, the mechanisms by which they may exert their plant-beneficial action are discussed.
Similar content being viewed by others
References
Belimov AA, Dodd IC, Hontzeas N, Theobald J, Safronova VI, Davies WJ (2009) Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signalling. New Phytol 181:435–447
Berg G, Eberl L, Hartmann A (2005a) The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ Microbiol 7:1673–1685
Berg G, Opelt K, Zachow C, Lottmann J, Götz M, Costa R, Smalla K (2005b) The rhizosphere effect on bacteria antagonistic towards the pathogenic fungus Verticillium differs depending on plant species and site. FEMS Microbiol Ecol 56:250–261
Berg G, Egamberdieva D, Lugtenberg B, Hagemann M (2010) Symbiotic plant–microbe interactions: stress protection, plant growth promotion and biocontrol by Stenotrophomonas. In: Seckbach JMG, Grube M (eds) Symbiosis and stress: joint ventures in biology, cellular origin, life in extreme habitats and astrobiology 17. Springer, Berlin, pp 447–463
Bloemberg GV, Lugtenberg BJ (2001) Molecular basis of plant growth-promotion and biocontrol by rhizobacteria. Curr Opin Plant Biol 4:343–350
Bloemberg GV, Lugtenberg BJJ (2004) Bacterial biofilms on plants: relevance and phenotypic aspects. In: Ghannoum R, O’Toole GA (eds) Microbial biofilms. ASM, Washington, pp 141–159
Brown MRW, Foster JHS (1970) A simple diagnostic milk medium for Pseudomonas aeruginosa. J Clin Pathol 23:172–177
Castric PA (1975) Hydrogen cyanide, a secondary metabolite of Pseudomonas aeruginosa. Can J Microbiol 21:613–618
Chin-A-Woeng TFC, Bloemberg GV, Mulders IHM, Dekkers LC, Lugtenberg BJJ (2000) Root colonization is essential for biocontrol of tomato foot and root rot by the phenazine-1-carboxamide-producing bacterium Pseudomonas chlororaphis PCL1391. Mol Plant Microbe Interact 13:1340–1345
Costacurta A, Vanderleyden J (1995) Synthesis of phytohormones by plant-associated bacteria. Crit Rev Microbiol 21:1–18
Crighton EJ, Elliott SJ, Upshur R, van der Meer J, Small I (2003) The Aral Sea disaster and self-rated health. Health Place 9:73–82
Dobbelaere S, Croonenborghs A, Thys A, Broek AV, Vanderleyden J (1999) Phytostimulatory effect of A. brasilense wild type and mutant strains altered in IAA production on wheat. Plant Soil 212:155–164
Egamberdieva D, Kucharova Z (2009) Selection for root colonizing bacteria stimulating wheat growth in saline soils. Biol Fertil Soi 45:563–571
Egamberdieva D, Kamilova F, Validov S, Gafurova L, Kucharova Z, Lugtenberg B (2008) High incidence of plant growth-stimulating bacteria associated with the rhizosphere of wheat grown on salinated soil in Uzbekistan. Environ Microbiol 10:1–9
Egamberdiyeva D, Gafurova L, Islam KR (2007) Salinity effects on irrigated soil chemical and biological properties in the Syr Darya basin of Uzbekistan. In: Lal R, Sulaimanov M, Stewart B, Hansen D, Doraiswamy P (eds) Climate change and terrestrial C sequestration in Central Asia. Taylor-Francis, New York, pp 147–162
FAO (2002) Crops and drops: making the best use of water for agriculture. FAO, Rome. Available at http://www.fao.org/docrep/w5146e/w5146e0a.htm
FAOSTAT (2007) FAOSTAT On-line. United Nations Food and Agriculture Organization, Rome. Available at http://faostat.fao.org/default.aspx
Glick BR, Penrose DM, Li JP (1998) A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theor Biol 190:63–68
Glick BR, Cheng Z, Czarny J, Duan J (2007) Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur J Plant Pathol 119:329–339
Haas D, Defago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3:307–319
Hankin L, Anognostakis SL (1977) Solid media containing carboxymethylcellulose to detect Cx cellulose activity of microorganisms. J Gen Microbiol 98:109–115
Howe TG, Ward JM (1976) The utilization of Tween 80 as carbon source by Pseudomonas. J Gen Microbiol 92:234–235
Jackson M (1997) Hormones from roots as signals for the shoots of stressed plants. Trends Plant Sci 2:22–28
Kamilova F, Validov S, Azarova T, Mulders I, Lugtenberg B (2005) Enrichment for enhanced competitive plant root tip colonizers selects for a new class of biocontrol bacteria. Environ Microbiol 7:1809–1817
Kamilova F, Kravchenko LV, Shaposhnikov AI, Azarova T, Makarova N, Lugtenberg BJJ (2006) Organic acids, sugars, and L-tryptophane in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol Plant Microb Interact 19:250–256
Kravchenko LV, Leonova EI, Tikhonovich IA (1994) Effect of root exudates of non-legume plants on the response of auxin production by associated diazotrophs. Microb Releases 2:267–271
Kravchenko V, Makarova NM, Azarova TS, Provorov NA, Tikhonovich IA (2002) Isolation and phenotypic characterization of plant growth-promoting rhizobacteria with high antiphytopathogenic activity and root-colonizing ability. Microbiology 71:521–525
Kravchenko V, Azarova TS, Leonova-Erko EI, Shaposhnikov AI, Makarova NM, Tikhonovich IA (2003) Root exudates of tomato plants and their effect on the growth and antifungal activity of Pseudomonas strains. Microbiology 72:48–53
Kravchenko L, Azarova TS, Makarova NM, Tikhonovich IA (2004) The effect of tryptophan present in plant root exudates on the phytostimulating activity of rhizobacteria. Microbiology 73:195–198
Kravchenko LV, Shaposhnikov I, Makarova NM, Azarova TS, Tikhonovich IA (2006) Dynamics of abundance of antifungal strains of Pseudomonas in the rhizosphere of hydroponic cucumbers grown on greenhouse mineral substrate. Microbiology 75:404–409
Kurth E, Cramer GR, Lauchli A, Epstein E (1986) Effects of NaCl and CaCl on cell enlargement and cell production in cotton roots. Plant Physiol 82:1102–1106
Leeman M, van Pelt JA, den Puden FM, Heinsbroek M, Bakker PAHM, Schippers B (1995) Induction of systemic resistance by Pseudomonas fluorescens in radish cultivars differing in susceptibility to Fusarium wilt, using a novel bioassay. Eur J Plant Pathol 101:655–664
Lifshitz R, Kloepper JW, Kozlowski M, Simonson C, Carlson J, Tipping EM, Zalesk I (1987) Growth promotion of canola (rapeseed) seedlings by a strain of Pseudomonas putida under gnotobiotic conditions. Can J Microb 33:390–395
Lugtenberg BJJ, Kamilova FD (2004) Rhizosphere management: microbial manipulation for biocontrol. In: Goodman RM (ed) Encyclopedia of plant and crop science. Marcel Dekker, New York, pp 1098–1101
Lugtenberg B, Kamilova F (2009) Plant growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556
Lugtenberg BJJ, Kravchenko LV, Simons M (1999) Tomato seed and root exudate sugars: composition, utilization by Pseudomonas biocontrol strains and role in rhizosphere colonization. Environ Microbiol 1:439–446
Lugtenberg BJJ, Dekkers L, Bloemberg GV (2001) Molecular determinants of rhizosphere colonization by Pseudomonas. Annu Rev Phytopathol 39:461–490
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci 166:525–530
Miller KJ, Wood JM (1996) Osmoadaption by rhizosphere bacteria. Ann Rev Microbiol 50:101–136
Okon Y, Itzigsohn R (1995) The development of Azospirillum as a commercial inoculate for improving crop yields. Biotechnol Adv 13:415–424
Okon Y, Bloemberg GV, Lugtenberg BJJ (1998) Biotechnology of biofertilization and phytostimulation. In: Altman A (ed) Agricultural biotechnology. Marcel Dekker, New York, pp 327–350
Raaijmakers JM, Paulitz CT, Steinberg C, Alabouvette C, Moenne-Loccoz Y (2009) The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321:341–361
Roberts P, Muchovej R, Achor D, Baker CA, Bruton B, Adkins S (2005) Investigation into a mature watermelon vine decline and fruit rot. Phytopathology 95:S89
Sakhabutdinova AR, Fatkhutdinova DR, Bezrukova MV, Shakirova FM (2003) Salicylic acid prevents the damaging action of stress factors on wheat plants. Bulg J Plant Physiol 21:314–319
Shirokova Y, Forkutsa I, Sharafutdinova N (2000) Use of electrical conductivity instead of soluble salts for soil salinity monitoring in Central Asia. Irr Drain Sys 14:199–205
Siddiqui A, Haas D, Heeb S (2005) Extracellular protease of Pseudomonas fluorescens CHA0, a biocontrol factor with activity against the root knot nematode Meloydogyne incognita. Appl Environ Microbiol 71:5646–5649
Smith D (1991) Growing pollution and health concerns in the Lower Amu Dar’ya basin, Uzbekistan. Sov Geogr 38:553–565
Spaepen S, Vanderleyden J, Okon Y (2009) Plant growth-promoting actions of rhizobacteria. In: van Loon LC, Ed Kader JC, Delseny M (eds) Adv Bot Res 51:283–320
Thomashow LS, Weller DM (1996) Current concepts in the use of introduced bacteria for biological disease control: mechanisms and antifungal metabolites. In: Stacey G, Keen NT (eds) Plant–microbe interactions. Chapman and Hall, London, pp 187–235
Van Loon LC (2007) Plant responses to plant growth-promoting bacteria. Eur J Plant Pathol 119:243–254
Walsh GA, Murphy RA, Killeen GF, Headon DR, Power RF (1995) Technical note: detection and quantification of supplemental fungal b-glucanase activity in animal feed. J Anim Sci 73:1074–1076
Werner JE, Finkelstein RR (1995) Arabidopsis mutants with reduced response to NaCl and osmotic stress. Physiol Plant 93:659–666
Wolf A, Fritze A, Hagemann M, Berg G (2002) Stenotrophomonas rhizophila sp. nov., a novel plant-associated bacterium with antifungal properties. Int J Evol Syst Microbiol 52:1937–1944
Zeng QY, Wang XR, Blomquist G (2003) Development of mitochondrial ssu rDNA-based oligonucleotide probes for specific detection of common airborne fungi. Mol Cell Probes 17:281–288
Zhang N, O’Donnell K, Sutton DA, Nalim FA, Summerbell RC, Padhye AA, Geiser DM (2006) Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J Clin Microbiol 44:2186–2190
Zholkevich VN, Pustovoytova TN (1993) The role of Cucumis sativum L leaves and content of phytohormones under soil drought. Russ J Plant Physiol 40:676–680
Zitter TA, Hopkins DL, Thomas CE (1996) Compendium of cucurbit diseases. APS, St Paul
Acknowledgments
This study was supported by an INTAS Collaborative project grant with Uzbekistan 04-82-6969 (coordinator BL) and by a UNESCO—L’OREAL Fellowship for “Women in Science” to DE.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Egamberdieva, D., Kucharova, Z., Davranov, K. et al. Bacteria able to control foot and root rot and to promote growth of cucumber in salinated soils. Biol Fertil Soils 47, 197–205 (2011). https://doi.org/10.1007/s00374-010-0523-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-010-0523-3