Abstract
Color vision is an important sensory capability that enhances the detection of contrast in retinal images. Monochromatic animals exclusively detect temporal and spatial changes in luminance, whereas two or more types of photoreceptors and neuronal circuitries for the comparison of their responses enable animals to differentiate spectral information independent of intensity. Much of what we know about the cellular and physiological mechanisms underlying color vision comes from research on vertebrates including primates. In insects, many important discoveries have been made, but direct insights into the physiology and circuit implementation of color vision are still limited. Recent advances in Drosophila systems neuroscience suggest that a complete insect color vision circuitry, from photoreceptors to behavior, including all elements and computations, can be revealed in future. Here, we review fundamental concepts in color vision alongside our current understanding of the neuronal basis of color vision in Drosophila, including side views to selected other insects.
Similar content being viewed by others
Introduction
In the life of insects and many vertebrates including humans, color vision is abundant and plays a central role in guiding behavior. It allows the discrimination of spectrally distinct stimuli regardless of their relative intensity. In humans, the perception of color is described by the attributes color hue, saturation, and brightness (Kelber and Osorio 2010; Lunau 2014), and it can be associated with additional sensations, emotions, or call up memories, explaining the importance of color for arts (Backhaus et al. 1998). How insects including Drosophila perceive color we cannot know. However, we can analyze and quantify the spectral discrimination abilities of insects in behavioral experiments, and we can refer to their color vision and presented color stimuli using the parameters spectral information, spectral purity, and intensity. Analyzing the underlying neuronal circuitries in insects and other taxa can provide us with a better understanding of the question whether the miniature brains of insects employ similar or different physiological, computational, and network mechanisms to derive information on spectral content as the much larger brains of, for instance, vertebrates (Dacey and Packer 2003; Gegenfurtner and Kiper 2003; Kelber et al. 2003; Gegenfurtner 2003; Solomon and Lennie 2007; Osorio and Vorobyev 2008; Jacobs 2008).
The observation that ‘simple organisms’ like insects are capable of color vision has been shown for the first time in the pioneering work of Karl von Frisch (von Frisch 1914). He conditioned honey bees to colored cardboards and showed that trained bees can distinguish the conditioned color from 30 different shades of gray. At this time, color vision was widely considered a privilege of certain vertebrates including humans, despite the many pollinating and frugivorous insects that obviously use spectral content to guide behavior. Today, we know that a wide range of animals from different taxa, among them numerous arthropods, are capable of color vision. Color vision endows these animals with extra power for the generation of contrast that facilitates image segmentation. The blossom of field cow-wheat that is frequently visited by different insects is difficult to recognize in the black and white image in Fig. 1a. However, addition of spectral information to the image makes the flower pop out from the green meadow (Fig. 1a) (colors are named according to human perception throughout the manuscript). Thus, color vision facilitates the identification of objects and enables a better judgement of their quality. The latter is demonstrated, for instance, by the coloration of fruits and flowers that often signal ripeness or that it is worth visiting a plant for its nectar (Fig. 1b). This includes changes in floral color that are exhibited by many angiosperm plants and that have been shown to instruct the behavior of pollinators (Weiss 1991).
Color vision facilitates image segmentation, object identification, and underlies diverse behaviors. a Addition of spectral contrast to the black and white image facilitates the segregation of objects from background. Field cow-wheat (Melampyrum arvense) pops out from the meadow when displayed in color. b Color vision enables a more accurate judgement of the properties of objects. For instance, floral color change can provide important cues for pollinators. After opening when flowers are still loaded with nectar, the shown Lantana (Lantana camara) flowers are yellowish. They change to orange and purple-red when nectar is increasingly depleted (Weiss 1991). c Color vision can enable intraspecific communication, also in the presence of co-occurring mimics. The wing patterns of Heliconius (Heliconius numata, upper left) and several closely related genera (Eueides isabella, lower right) display a shared warning signal. Yellow pigmentation in Heliconius numata with additional reflection in the UV, and additional UV sensitivity are consistent with a trait for intraspecific communication (Bybee et al. 2012). d Color vision can enable the detection of wing interference patterns (WIPs) that are displayed by the wings of most Hymenoptera and Diptera (here Drosophila melanogaster). WIPs have been suggested to serve intraspecific communication and were recently shown to be an important trait in sexual selection behavior in Drosophila (Shevtsova et al. 2011; Hawkes et al. 2019). e Insect color vision with sensitivity in the UV range of the spectrum, in addition to sensitivity for longer wavelengths, allows many insects to detect patterns on flowers that are hidden to the human eye. A buttercup flower (Caltha palustris) is perceived homogeneous yellow by a human observer (left) although it strongly reflects in the UV range (right, photographed with a 310–390 nm filter and displayed in greyscale). Images in (c) modified, Bybee et al. 2012 (d) Shevtsova et al. 2011; (e) modified, © Dr Schmitt, Weinheim Germany, uvir.eu
Next to this important function in the identification of sources of food, color vision can have an important role in the identification of predators, conspecifics and in communication (Poulton 1890; Osorio and Vorobyev 2008; Lunau 2014; Osorio and Cuthill 2015; Cuthill et al. 2017). For instance, Heliconius butterflies display a warning wing pattern that is shared among different local butterfly species. Yellow color patches with additional reflection in the UV range and additional rhodopsin molecules that provide extra sensitivity in the UV range in Heliconius numata have been interpreted in the context of intraspecific communication (Kronforst et al. 2006; Bybee et al. 2012) (Fig. 1c). Also, Diptera and Hymenoptera display colorful wing patterns (Fig. 1d; Shevtsova et al. 2011). Two recent studies, one on Drosophila melanogaster and one on Drosophila simulans demonstrate that these wing interference patterns serve intraspecific communication and sexual selection behavior (Katayama et al. 2014; Hawkes et al. 2019).
The wavelengths contributing to color vision and behavior strongly depend on the types of photoreceptors and their spectral sensitivities. The buttercup flower is uniform yellow for a human observer (Fig. 1e, left). Its UV reflectance (Fig. 1e, right) is not detected by the short (S), middle (M) and long (L) wavelength-sensitive cones that govern color vision in humans, and that have likely evolved to solve other tasks, including the detection of reddish fruits against green foliage (Mollon 1999; Regan et al. 2001; Melin et al. 2013). In contrast, the contained reflection in the UV range is very well visible to many insect pollinators that have photoreceptors with spectral sensitivity in the UV range (Menzel and Backhaus 1991; Briscoe and Chittka 2001; Chen et al. 2013; Lunau 2014). This example highlights that the detection of color depends on who is looking. The spectral properties of photoreceptors and the exact neuronal mechanisms for the comparison of their signals determine the range and type of spectral contrast that can be detected. Thereby, subtle differences in the properties of the color vision system can decide whether or not small differences in the spectral advertising strategies of flourishing plants can be detected, as recently shown for a mixed community of local bee pollinators and their preferred plants (Shrestha et al. 2019).
In local communities of pollinating insects and plants, the wavelengths where sharp changes in the reflectance of flowers occur can correspond well with the wavelengths where pollinators have maximum wavelength discrimination ability, as shown for instance, for different plant–bee communities (Chittka and Menzel 1992; Dyer et al. 2012). In this sender–receiver interplay, it is difficult to judge whether also the visual capabilities of the pollinators influence the floral color display. Using spectral analysis of flower signals and models of insect color vision, this has recently been suggested for a plant–pollinator community with reduced diversity of floral visitors. On a southern ocean island, with flies as the only pollinators, the chromatic capabilities of the fly visual system seemingly impose a filter on floral color display and plant community assembly (Shrestha et al. 2016).
In summary, colored traits and color detection are of great importance in the life of many insects (Hempel de Ibarra et al. 2014; Cuthill et al. 2017; Lebhardt and Desplan 2017). In contrast to bees or butterflies, however, we know relatively little about the ecological functions of color vision in Drosophila. Further studies, particularly in its natural habitat that could be related to the behavioral studies under laboratory conditions, would be very valuable (Dickinson 2014).
Photoreceptor opponency—a hallmark of color vision
Color vision is the result of sequential processing stages. In the first stage of color processing, photoreceptors report spectral and intensity changes of light as a change in membrane potential. Hereby, the spectral sensitivity of a given photoreceptor determines its probability to absorb a photon with a certain wavelength. The photoconversion of light energy during phototransduction (revealed in Drosophila in great detail and reviewed in Hardie 2001; Hardie and Juusola 2015) is independent of the wavelength of the absorbed photon(s). The information on the wavelength of a photon is lost in the moment it is absorbed by the photoreceptor. An increase in the photoreceptor response can similarly arise from an increase in light intensity or from the wavelength of the stimulus getting closer to the wavelength of maximal sensitivity of the photoreceptor (Fig. 2a). Consequently, a visual system with a single type of photoreceptor cannot discriminate the spectral property of a stimulus from its intensity. Numerous physically different stimuli can elicit same responses (Fig. 2a and a’). These circumstances are the key predictions of the ‘principle of univariance’ (Rushton 1972).
Photoreceptor functions, color opponent processing and photoreceptor sensitivity in selected insects. a, a′ Principle of univariance: light stimuli S1, S2, and S3 differ in wavelength and intensity (a), but elicit identical responses in a given photoreceptor (a´). b Example for a dichromatic color vision system with short and long wavelength-sensitive photoreceptors. b′ The metameric light stimuli (S1 + S1*) and S2 elicit same response in the two types of photoreceptors and are therefore interpreted as same color. c Neuronal response of a hypothetic color opponent neuron that receives antagonistic input from the two types of photoreceptors in (b). d Spectral sensitivities of the three types of photoreceptors in the trichromatic honey bee visual system (peak sensitivity in the UV, blue, and green range of the spectrum). e Spectral sensitivities of the five types of rhodopsins expressed in the predominating types of photoreceptors of the Drosophila eye (maximum sensitivity at 478 nm (Rh1, gray), 345 nm (Rh3, light purple), 375 nm (Rh4, violet), 437 nm (Rh5, blue), or 508 nm (Rh6, green). An accessory pigment mediates additional UV sensitivity in R1–R6. f Spectral sensitivities of the six classes of spectral receptors of Papilio xuthus: UV, violet, blue, green (double-green depicted), red and broad-band. Images modified, after (d) Osorio and Vorobyev (2008); (e) Salcedo et al. (1999) and Schnaitmann et al. (2018), and (f) Arikawa (2017)
In the second stage of color processing, the responses of different types of photoreceptors with different spectral sensitivities are compared by the nervous system. In this process, two, three or more different types of photoreceptors do per se not overcome the limitations imposed by the principle of univariance. Multiple receptor classes are not sufficient but necessary for color vision. It is the comparison of their activities that provides information about the spectral properties of a visual stimulus (Kelber and Osorio 2010; Mollon 1999). Thus, in its ‘simplest’ form, color vision is dichromatic and employs two types of spectrally distinct photoreceptors (Fig. 2b, b′). Summation of their output yields a measure of absolute intensity, and calculation of the difference between the responses of both types of photoreceptors enables the encoding of spectral information. The resulting color opponent responses have been observed in second- or higher-order postsynaptic neurons that are excited at one wavelength and inhibited at another (Fig. 2c). However, fingerprints of color opponent processing can already be detected in certain photoreceptors and in particular in the presynaptic terminals of photoreceptors (see ‘circuit mechanisms’). In the spectral range where the calculated difference between the photoreceptor sensitivities has its maximum slope, the power of animals to discriminate wavelength differences is often the highest. Still, two different photoreceptor types can be stimulated to the exact same extent by a wide range of metameric stimuli with very different wavelength composition (Fig. 2b, b’). Such metameric stimuli cannot be discriminated by the observer. This ambiguity can be reduced by employing more types of photoreceptors. The number of naturally occurring spectral signals that can elicit identical responses in different photoreceptor types decreased with increasing number of photoreceptors types (Osorio and Vorobyev 2008). The importance of color opponent processing for color vision can hardly be overrated (Chittka et al. 1992; Dacey and Packer 2003; Gegenfurtner and Kiper 2003; Jacobs 2014; Demb and Singer 2015). In fact, the concept of color opponency dominates the way how researchers think about color vision since Hering published his opponent color theory more than 100 years ago. Ever since, the search for individually identifiable neurons that exhibit color opponent processing became an important aspect of color vision research (Hering 1878; Backhaus et al. 1998; Jacobs 2014).
In insects, the diversity of photoreceptors can vary considerably from one species to another. For instance, the visual systems of cockroaches and many ant species have two types of spectrally different photoreceptors (Mote and Goldsmith 1970; Yilmaz et al. 2017). Vision in honey bees involves photoreceptors that are most sensitive in either the UV, blue, or green range of the spectrum (Fig. 2d). In Drosophila and a number of other flies, the visual system harbors five photoreceptor types (Fig. 2e). An even larger diversity is found in the butterfly Papilio xuthus with a retina containing six ‘classes’ of photoreceptors (Fig. 2f) or the bluebottle butterfly Graphium sarpedon that even has 15 types of photoreceptors (Chen et al. 2016).
Does color vision in these animals necessarily involve all photoreceptor types? And does a higher grade of spectral photoreceptor diversity in an animal correlate with better color vision? Experiments comparing trichromatic RGB devices and hyperspectral cameras demonstrate that multiple, narrow-band sensors enable better reconstructions of a given spectrum (Garcia et al. 2015). In biological systems, however, narrow bandwidth photoreceptors come at a cost of a low signal-to-noise ratio (van Hateren 1993; Osorio and Vorobyev 2008). Also, increased diversity of both photoreceptors and neural circuits that mediate their comparison increases the metabolic and other costs of every bit of encoded spectral information (Niven and Laughlin 2008). Thus, the question arises how many types of different photoreceptors with what kind of spectral tuning properties (shape and width) are optimal for color vision. Studies that used spectra of natural scenes with assumed ecological relevance and models of color vision in different animals suggest that the encoding of spectral information is not improved by more than five types of photoreceptors (reviewed in Osorio and Vorobyev 2005). Thus, the function of higher photoreceptor diversity in color vision remains an open question. In some animals, specific photoreceptor types might drive wavelength-specific behavior (Kelber et al. 2003; Kelber and Osorio 2010).
Detailed insights into the diversity of insect retinae and the contribution of photoreceptors to behaviors guided by spectral cues have been revealed (Kelber et al. 2003; Kelber and Osorio 2010; Hempel de Ibarra et al. 2014; Arikawa 2017). However, our understanding of the molecular mechanisms, physiology, and circuit implementation of color vision in insects is still limited. Help might come from Drosophila where advances in genetic targeting of single cell types, anatomical, physiological, perturbational, and behavioral investigation open new avenues to address these questions.
The Drosophila retina and optic lobe
Drosophila, as most other insects, possesses two compound eyes, each equipped with 750–800 ommatidia. In other insect species, the number of ommatidia per eye can be as low as a dozen and up to several thousand (Lunau 2014). Each individual ommatidium of Drosophila houses eight photoreceptors, which is common to Diptera and most other insects. Specific combinations of photoreceptor types give rise to different types of ommatidia. Drosophila ommatidia house six outer photoreceptors R1–R6 (short visual fibers, svfs) that project to the first optic neuropil, the lamina, and a pair of superimposed inner R7 and R8 photoreceptors (long visual fibers, lvfs) that project to the second optic neuropil, the medulla (Fig. 3a). Gene regulatory networks and a mix of local signaling events and stochastic mechanisms specify photoreceptor cell fate and give rise to the three ommatidial types of the main part of the Drosophila eye (reviewed in Johnston 2013; Mikeladze-Dvali et al. 2005). The large majority of ommatidia belong to the yellow (y) and pale (p) subtype that are randomly distributed at a ratio of roughly 2:1 over the retina (Franceschini et al. 1981; Chou et al. 1996). In Drosophila, ommatidia usually follow the ‘one photoreceptor–one rhodopsin’ rule and rhodopsin expression in R7/R8 is tightly coupled: R7p and R8p express rh3 and rh5 with maximum sensitivities in the short-UV and blue spectral range, respectively. R7y and R8y express rh4 and rh6 with maximum sensitivity in the long-UV and green spectral range, respectively (Salcedo et al. 1999; Figs. 2e,3a). Yellow ommatidia in the dorsally oriented third of the retina (dorsal-yellow, dy) break with the ‘one photoreceptor—one rhodopsin’ rule (Mazzoni et al. 2008). In dy ommatidia, R8 express rh6 as in y ommatidia, but R7 co-express rh4 and rh3 (Fig. 3a). In addition to the three types of ommatidia in Drosophila, there are ommatidia specialized in the detection of polarized light in the dorsal-most retina, the so-called dorsal rim area (DRA), that are also present in many other insects (reviewed in Mathejczyk and Wernet 2017). Also in honey bees and Papilio xuthus, different combinations of photoreceptors establish three types of ommatidia (Fig. 3b, c). Notably, it has been shown recently that two butterfly species employ gene regulatory mechanisms that resemble mechanisms in Drosophila to specify the random ommatidia mosaik (Wernet et al. 2006; Perry et al. 2016).
Schematic representation of photoreceptor composition in the predominating types of ommatidia in the Drosophila, honey bee, and butterfly (Papilio xuthus) eye. a In Drosophila, rhodopsin expression in the long visual fibers (lvfs) R7/R8 differs in yellow (y), dorsal-yellow (dy, in the dorsal third retina), and pale (p) ommatidia. R7p/R8p express rh3/rh4 (light purple/blue), R7y/R8y express rh4/rh6 (dark purple/green), and dy R8/R7 express rh6 and rh3 + rh4. Short visual fibers (svfs) R1–R6 homogeneously express rh1 (Salcedo et al. 1999). b In Apis mellifera, opsin expression in the two lvfs determines three main ommatidia types. In type I, one lvf expresses UV sensitive (light purple), the other blue (blue) sensitive opsin. Both lvfs express UV sensitive opsin in type II, and blue sensitive opsin in type III ommatidia. Short visual fibers uniformly express green sensitive opsin in all ommatidia. The sensitivity and function of the small R9 is unknown (Wakakuwa et al. 2005). c In Papilio xuthus, UV and blue sensitive opsin expression in the lvfs of type I–III ommatidia is similar as in bees. In all ommatidia types, two svfs co-express two long wavelength-sensitive opsins providing them with maximum sensitivity to green light. The remaining four svfs express red sensitive opsin in type I, red plus green sensitive opsins in type II, and green sensitive opsin in III. Furthermore, spectral sensitivity of Papilio photoreceptors is modulated by red (type I and II) or yellow (type III) perirhabdomal pigments and ‘fluorescence pigment’ (type II) (Arikawa 2017). The sensitivity of small R9 is unknown. In the neuronal superposition eye of Drosophila, the individual rhabdomeres (gray in a) are spatially and optically separated. In contrast, bee and butterfly ommatidia have a so-called fused rhabdom, where the light-sensitive structures of the individual photoreceptors are grouped closely together and acts as a light guide
In contrast to bees and butterflies that have a so-called fused rhabdom in which the light-sensitive rhabdomeres are in close proximity and act as a single light guide with a shared optical axis, the rhabdomeres of Drosophila’s R1–R6 stay optically isolated from each other (Fig. 3). Only the rhabdomeres of the central photoreceptors R7 and R8 form an optical unit. In this ‘light guide’, R7 filters the light before the remaining light reaches R8 (Trujillo-Cenóz and Melamed 1966; Braitenberg 1967). Furthermore, in Diptera and few other insects (Lunau 2014), one outer photoreceptor of each of six neighboring ommatidia and one pair of R7/R8 from a seventh ommatidium receive light from the same location in visual space. Their axons converge in 1 of about 750 cartridges of the lamina, visual sampling units that reflect both the ommatidial organization of the compound eye and the wiring of ‘neuronal superposition’ eyes (Kirschfeld 1973; Langen et al. 2015). Other than in butterflies and bees, where synaptic interactions in the lamina are likely involved in color opponent processing or in increasing wavelength specificity (Takemura et al. 2005; Chen et al. 2013), lvfs in Drosophila pass the lamina without making synapses (Fig. 4, Chen et al. 2019; Meinertzhagen and O’Neil 1991; Menzel and Backhaus 1991; Menzel and Blakers 1976; Ribi 1981; Takemura and Arikawa 2006). The terminals of R7 and R8 in Drosophila are often described to ‘precisely’ terminate in medulla layer m6 and m3, respectively (Fig. 4a; Takemura et al. 2008), but reconstructions based on serial electron microscopic data show that R7 forms chemical synapses in m6 and all distal layers, and R8 in m1–m3 (Takemura et al. 2013).
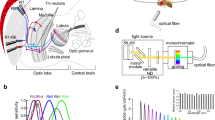
Neuronal basis of color vision in Drosophila. a Anatomical representation of demonstrated and candidate color processing neurons in the fly optic lobe. Retina (Re), lamina (La), medulla (Me), lobula (Lo), and lobula plate (Lop). b R7 and R8 photoreceptors of the same type of ommatidia mutually inhibit each other directly via HisCl1 histamine receptors and receive additional feedback inhibition via Dm9 that requires the second histamine receptor Ort (Schnaitmann et al. 2018; manuscript in preparation). c Connectivity and suggested function of the cells in (a). The axons of R7 and R8 pass through the lamina and convey information to the distal layers m1–m6 of the medulla. Transmedulla neurons Tm5a, b, c and Tm20, but also amacrine cells including Dm8 receive direct input from R7 or R8. R7 and R8 terminals mutually inhibit each other [see (b)]. The svfs of the outer photoreceptors R1–R6 convey information to the lamina monopolar cells L1–L3 that in turn project to the medulla. Simultaneous block of L1 and L2 prohibited blue–green discrimination in a memory task (Schnaitmann et al. 2013). L1–L3 connect to a range of different Tms, among them some with a function in color vision (Tm20 for L2 and L3, Tm5a for L3) (Gao et al. 2008; Takemura et al. 2015). Tm5a,b,c, and Tm20 establish redundant channels of the color vision system (Melnattur et al. 2014). The R7 → Dm8 → Tm5c pathway and the medulla columnar neuron MC61 are necessary for UV/green preference behavior (Gao et al. 2008; Otsuna et al. 2014; Karuppudurai et al. 2014). Tms relay information to the Lobula, for instance, to the lobula intrinsic neuron Li4 and the visual projection neuron LT11 (not shown, Otsuna et al. 2014; Lin et al. 2016). VPN–MB1 establish a direct link from the medulla to the mushroom body, and are necessary for color discrimination in a memory task (Vogt et al. 2016). Round endings and arrowheads denote inhibitory (histamine) and excitatory connections, respectively. Dashed lines indicate unspecified connectivity. Image in (a) after Fischbach and Dittrich (1989)
In each lamina cartridge, the signals from R1–R6 are conveyed to the large lamina monopolar cells L1–L3 and to a lamina intrinsic amacrine cell (Meinertzhagen and O’Neil 1991). Eleven different cell types of each cartridge connect the lamina with the medulla, the lamina monopolar cells L1–L5 that send their axons to the medulla, the centrifugal cells C2 and C3 that send their axons to the lamina, T1, Lawf1, and Lawf2 cells (reviewed in Borst et al. 2010; Tuthill et al. 2013). The medulla is the first optic neuropil that receives signals from photoreceptors with different spectral sensitivities and it harbors color vision circuitries (see ‘circuit mechanisms’). In addition to the retinotopic projections of the lamina neurons and the terminals of the lvfs R7 and R8, each medulla column (visual sampling unit of the medulla) houses more than 60 different cell types (Fischbach and Dittrich 1989; Takemura et al. 2013, 2015). Among these are local interneurons including multi-columnar distal medulla (Dm) cell types that laterally connect medulla columns, and transmedulla (Tm) neurons that connect the medulla to the lobula or the central brain. Different cell types directly postsynaptic to R7 and R8 have been identified by genetic labeling techniques and serial electron microscopic reconstructions (Fig. 4a) (Gao et al. 2008; Takemura et al. 2013; Karuppudurai et al. 2014; Takemura et al. 2015). Insights into the contribution of some of these cell types to neuronal circuitries underlying behavior guided by spectral cues are described in detail in our section on circuit mechanism (below). The importance of the medulla and lobula for color vision in insects is generally supported by the observation of color opponent neurons in theses neuropils in different bees and butterflies (Swihart 1972; Kien and Menzel 1977a, b; Hertel 1980; Yang et al. 2004; Paulk et al. 2008, 2009a). The circuitries of the lobula plate have so far not been linked to spectral processing. They play an important role in the computation of visual motion and navigation (see review by Borst et al. 2019 in this issue).
Behavior guided by spectral cues
In Drosophila, dual choice assays were employed to analyze the innate preference of walking flies for monochromatic light against darkness (‘phototaxis’), and to analyze how flies choose between two spectrally distinct lights (‘differential phototaxis’ or ‘spectral preference’). Both paradigms revealed positive phototaxis from 250 to 650 nm with two maxima, one in the blue and one in the UV wavelength range (Bertholf 1932; Fingerman and Brown 1952; Schümperli 1973; Harris et al. 1976; Hu and Stark 1977). When testing phototaxis to UV light of increasing intensity, Drosophila preference increases until it saturates at medium intensities. With green light, preference similarly increases up to medium intensities, but decreases and even turns into avoidance at higher intensities (Jacob et al. 1977; Fischbach 1979; Gao et al. 2008). Thus, in Drosophila, phototactic behavior is not purely achromatic as it does not exclusively correlate with the intensity of light. Instead, it also depends on the wavelength of presented light and can therefore not be the result of a simple summation of photoreceptor responses. A dependence of phototaxis on the spectral composition of light has further been observed when flies could choose between UV and a mixture of green and UV light (same intensity of UV in both stimuli, i.e. pure UV stimulus in all experiments is darker than the mixture). In these experiments, flies preferred the spectrally mixed stimulus at low UV intensities and the pure UV stimulus at high UV intensities (Heisenberg and Buchner 1977; Fischbach 1979). Altogether, phototaxis in Drosophila involves both chromatic and achromatic discrimination. Differentiating between the two in spectral preference experiments with monochromatic stimuli has hitherto not been feasible.
The ecological function of the observed phototactic behavior of Drosophila is not known, it may serve orientation behavior towards open space. This assumption largely rests on fact that UV is typically more prominent in the open sky compared with UV reflectance from objects in the ventral half of the field of view (Möller 2002). In line with this assumption, female Drosophila orient towards UV light when there is no demand to lay eggs. However, to lay eggs, flies turn away from UV light to lay them on dark substrate (Zhu et al. 2014; Guntur et al. 2017). Thus, phototaxis can depend, at least in mated females, on the internal state. A recent study furthermore suggests that phototaxis in addition depends on the time of the day (Lazopulo et al. 2019). This was observed when analyzing spectral preference of Drosophila between blue, red, and green illuminated areas using light intensity levels much higher than in all previous studies. Under these conditions, Drosophila showed a strong preference for green in the early morning and late afternoon, reduced preference for green at midday and robust avoidance of blue throughout the day (Lazopulo et al. 2019). It is still somewhat unclear whether these two behaviors (UV avoidance in the context of egg laying and the change of spectral preference during the course of the day) depend on the analysis of spectral content. In honey bees, phototaxis solely depends on the intensity of light (Kaiser et al. 1977; Menzel and Greggers 1985) (but see discussion below). In contrast, many innate behaviors that involve color vision have been reported in insects (Kelber et al. 2003). For instance, the butterflies Papilio aegeus and Pieris brassicae prefer to oviposit on green substrate, the hoverfly Eristalis tenax prefers to feed on small yellow objects and the ant Camponotus blandus exhibits a strong innate preference for UV over blue and green stimuli (Lunau and Wacht 1994; Kelber 2001; Yilmaz et al. 2017).
Color vision in Drosophila has also been studied using color conditioning paradigms in which the animals were trained to associate a color with either reward or punishment (Menne and Spatz 1977; Kelber et al. 2003; Tang and Guo 2001; Schnaitmann et al. 2010, 2013; Melnattur et al. 2014). In differential conditioning, one spectral stimulus is presented together with, for instance, sugar reward, while another spectral stimulus is presented without. After several rounds of training, flies can choose between the two color stimuli. If flies show a preference for the previously rewarded stimulus, one can infer that they can discriminate the two visual stimuli. Compared with spectral preference experiments, color conditioning greatly facilitates the analysis of intensity invariant stimulus discrimination in Drosophila. Changing or reversing the relative intensities of the visual stimuli between training and test in differential conditioning experiments allows to test whether the flies’ choices are based on spectral properties or the intensity of the visual stimuli (Menne and Spatz 1977; Tang and Guo 2001; Schnaitmann et al. 2013; Melnattur et al. 2014). Using differential color conditioning, a wavelength discrimination function (Δλ/λ) was determined for Drosophila with two wavelength ranges where the capability to discriminate neighboring wavelengths is highest. In the violet (≈ 420 nm) and in the blue–green (≈ 495 nm) wavelength range, flies can discriminate stimuli that differ about 20 nm in wavelength (Hernández de Salomon and Spatz 1983). In their important pioneering study, Hernández de Salomon and Spatz (1983) tested only relatively few wavelengths and the missing analysis of UV excludes major sensitivities of the Drosophila compound eye (Fig. 2e). In addition, they used spectral preference behavior to reveal isoluminant stimuli. This approach is questionable because spectral preference itself depends on both spectral and intensity properties of stimuli. Thus, a potential contribution of intensity discrimination cannot be excluded. When compared with bees or butterflies, the obtained Δλ/λ values for Drosophila are rather large. Differential color conditioning experiments in freely flying honey bees revealed a Δλ/λ function with two optima, one in the violet (≈ 400 nm) and one in the blue–green (≈ 500 nm) wavelength range. However, bees were able to discriminate stimuli differing only 4–6 nm in wavelength (von Helversen 1972). An even more accurate and complex color discriminability was found in the butterfly Papilio xuthus. Using proboscis extension reflex (PER) conditioning (Koshitaka et al. 2008), the Δλ/λ function exhibits three optima at 420, 480 and 560 nm, respectively, and butterflies were able to discriminate stimuli as close as 1 nm apart from each other.
Importantly, color discrimination in Drosophila strongly depends on the precise design of the behavioral assay, and whether flies are agitated or undisturbed prior to behavioral decisions (Harris et al. 1976; Heisenberg and Buchner 1977; Jacob et al. 1977; Fischbach 1979; Gao et al. 2008). Dependencies on the behavioral assay were also observed in bees. Investigation of wavelength discrimination using either the proboscis extension reflex in restrained bees or behavioral decisions in freely moving bees as readout revealed much better wavelength discrimination in the latter (Niggebrügge et al. 2009). Also, differential conditioning yields better discrimination than absolute conditioning in bees (Giurfa 2004). If spectral discrimination in Drosophila also depends on the angular size of the presented stimuli remains unknown. In bees, discrimination relies exclusively on achromatic L-receptor contrast if stimuli are small (5°–15° of visual angle), whereas larger stimuli are discriminated exclusively based on chromatic contrast (Giurfa et al. 1996, 1997; Giurfa and Vorobyev 1998; Ng et al. 2018). Thus, more than ~ 60 neighboring ommatidia must be stimulated to enable object detection based on chromatic mechanisms in bees. In contrast, stimulation of only seven ommatidia enables object detection based on L-receptor contrast. These findings might also provide a simple explanation for experiments in which bee phototaxis behavior was found to be achromatic: the presented stimuli of 1–9.5° of angular size might have been too small to enable chromatic processing mechanisms to become active (Kaiser et al. 1977; Menzel and Greggers 1985). With respect to color vision research in Drosophila, it is important to note that spatial color contrast and color constancy, that are well documented in at least some insects including butterflies and bees have not been reported so far (reviewed in Chittka et al. 2014). In contrast, temporal color contrast is detected by Drosophila (Fischbach 1979).
Photoreceptor contributions to color vision
In the 1960s, Seymour Benzer pioneered the field of neurogenetics by isolating Drosophila mutants with deficits in phototaxis using chemical mutagenesis (Benzer 1967). Later, further mutants with impaired vision were isolated based on noticeable defects in electroretinogram recordings (Koenig and Merriam 1977). Specific structural and functional defects were assigned to in particular the mutants rdgBKS222, sevLY3 and oraJK84 that were used to reveal the contribution of photoreceptors to phototaxis and spectral preference (Harris et al. 1976; Heisenberg and Buchner 1977; Hu and Stark 1977; Jacob et al. 1977; Fischbach 1979). In oraJK84 and rdgBKS22, R1–R6 completely degenerated in the presence of light. In sevLY3, mutant R7 photoreceptors are completely absent due to a developmental defect. Studies based on these mutants suggested that all photoreceptors can contribute to phototaxis and spectral preference. However, it became clear that their contributions critically depend on the exact stimulus conditions. Spectral preference behavior to two monochromatic stimuli, with one as a constant reference and the other varied in intensity and wavelength, is mainly mediated by R7 and R8 (Hu and Stark 1977). In contrast, when spectral preference is tested with a ‘white’ reference stimulus, it involves all photoreceptor types (Schümperli 1973; Harris et al. 1976). Phototaxis experiments also revealed that R1–R6 have a higher absolute light sensitivity than R7/R8 (Harris et al. 1976; Jacob et al. 1977).
Years later, it was shown that sevLY3 causes uniform rh6 expression in all R8 photoreceptors in addition to the known R7 degeneration (Chou et al. 1999). oraJK84 turned out to be a double mutant with null mutations in ninaE (rh1) and ort (O’tousa et al. 1989). The previously unnoticed mutation in ort affects one of two histamine receptor genes of the Drosophila genome that is expressed widely in the fly visual system in all neurons downstream of all types of photoreceptors (Gao et al. 2008). Thus, the ort mutation included in oraJK84 strongly affects visual processing, in addition to the known absence of R1–R6 function. More recent studies that employed either specific mutations in individual rhodopsin genes or specific rhodopsin promoters to drive block of synaptic transmission in specific photoreceptor types overcome these problems. However, despite significant differences in the specificity of the approaches, the new studies largely corroborated the major findings of older work and demonstrated that each photoreceptor type (R1–R6, R7, R8) can drive spectral preference on its own (Gao et al. 2008; Yamaguchi et al. 2010).
Few studies addressed whether individual photoreceptor classes contribute to the chromatic aspect of phototactic behavior. Color mixing experiments revealed that sev mutants did not exhibit the wavelength dependent reactions to mixed UV/green stimuli that were exhibited by wild-type flies (see ‘behavior guided by spectral cues’, Heisenberg and Buchner 1977; Fischbach 1979). Furthermore, sev mutant flies did not show the negative phototaxis at high intensities of green light (Jacob et al. 1977). These findings suggest that R7 has a prominent role in the chromatic aspect of phototactic behavior.
Based on their broad-band sensitivity and major role in motion vision (Heisenberg and Buchner 1977; Yamaguchi et al. 2008; Wardill et al. 2012), R1–R6 were thought not to contribute to fly color vision (Troje 1993). This view was challenged by the finding that Drosophila color discrimination is described best using a receptor noise-limited model that incorporates R1–R6 (Hernández de Salomon and Spatz 1983; Schnaitmann et al. 2013; Garbers and Wachtler 2016). Most importantly, flies with R1–R6 and R7y as only functional photoreceptors are able to discriminate blue and green (Schnaitmann et al. 2013). This finding rests on experiments combining genetic interference with photoreceptor function and appetitive color conditioning. However, it turned out that R1–R6 are not required in general for this specific spectral discrimination: flies with function restricted to R7y and R8y were also able to discriminate blue and green, whereas flies with function restricted to R1–R6 and R8y failed. These experiments suggest that fly color vision involves comparisons between R1–R6 and R7y as well as R7y and R8y. This conclusion is in line with the involvement of R7 in the chromatic aspect of phototaxis (see above). Flies with function restricted to R1–R6, R7p and R8p were not able to discriminate blue and green. To further explore the role of photoreceptor types in Drosophila color vision, the range of the wavelengths that are analyzed in spectral discrimination tasks must be extended. In particular, it remains to be addressed whether also the photoreceptors of pale ommatidia are involved in the discrimination of shorter wavelengths, as suggested by work in Lucilia (Troje 1993).
Color discrimination in bees involves all photoreceptor types, and computational models including all three photoreceptor types explain behavioral observations well (Neumeyer 1980; Backhaus et al. 1987; Backhaus 1991; Chittka 1992; Brandt and Vorobyev 1997; Vorobyev et al. 2001). Electrophysiological recordings in the bee visual system revealed a large diversity of different color opponent response types, and modeling work demonstrates that all three photoreceptor types are involved (Kien and Menzel 1977a, b; Hertel 1980; Yang et al. 2004; Paulk et al. 2008, 2009a, b; Vasas et al. 2019). In contrast, color discrimination in Papilio xuthus does not involve all of its six ‘spectral classes’ of photoreceptors (Koshitaka et al. 2008). The Δλ/λ function of these butterflies exhibits three optima that are interpreted as evidence for three photoreceptor opponent computations in color vision. A receptor noise-limited color opponency model including UV, blue, green, and red photoreceptor sensitivities yields the best fit to the Δλ/λ function (Koshitaka et al. 2008; Arikawa 2017).
Circuit mechanisms underlying color vision
Physiological studies that include detailed investigations of the identity, function and nature of synaptic and circuit interactions in insect color vision are still rare. Until recently, this included the physiology of insect photoreceptor terminals that were uncharted territory (see below). Intracellular recordings from distal segments of photoreceptors of many insect species showed positive membrane potential changes; only few studies reported additional evidence for spectral inhibition. Seldom encounters of additional negative interactions have been reported in bees where some of the recorded UV receptors depolarized at short wavelengths and hyperpolarized at wavelengths longer than 420 nm. Comparable hyperpolarizing responses have been observed in some UV- and B-receptors in bumble bees (Skorupski et al. 2007). Why such hyperpolarizing responses were only observed in few of the recordings remained unknown. Most photoreceptors were excited over a wavelength range broader than expected from rhodopsin sensitivity (Menzel and Blakers 1976). Moreover, the spectral inhibition was absent when recordings were made in axons in the lamina instead of outer photoreceptor segments. These axonal recordings revealed ‘pure single pigment spectral sensitivity’, which led the authors to conclude that positive and negative interactions cancel each other (Menzel and Blakers 1976).
Clear evidence for inhibitory photoreceptor interactions in insects comes from experiments in few butterfly species where a fraction of the recorded photoreceptors exhibited hyperpolarizing responses to light (Horridge et al. 1983; Matić 1983; Chen et al. 2013). In these intracellular recordings, the reference electrode was usually placed in the body cavity and Matić (1983) showed that many of the hyperpolarizing interactions were strongly reduced or turned into excitation when the reference electrode was placed in the extracellular space close to the recording electrode. He explained this observation by the presence of strong light-induced fluctuations of the extracellular potential of up to 60 mV (Matić 1983). However, because inhibition was retained in some of the recordings, Matić concluded that inhibition between photoreceptor is not a methodological artifact and that butterflies make a special case.
New insights came from a recent study in Drosophila. Two-photon calcium imaging with subcellular resolution revealed UVshort vs. blue and UVlong vs. green opponent processing in the terminals of R7p/R8p and R7y/R8y, respectively (Schnaitmann et al. 2018). These first color opponent interactions in the fly visual system rely on mutual inhibition between R7 and R8 of either type of ommatidia. Notably, this spectral inhibition is mediated by two mechanisms that act in parallel. First, the Drosophila histamine-gated chloride channel HisCl1 is expressed in inner photoreceptor terminals and mediates direct reciprocal synaptic inhibition between R7 and R8 of the same ommatidium (mutual inhibition within a single column). Second, R7 and R8 receive spectral feedback signals from neurons in the medulla that express the second Drosophila histamine receptor Ort, in particular from the multi-columnar Dm9 cell as revealed in recent work of our laboratory (Fig. 4b) (Schnaitmann et al. 2018; manuscript in preparation). These two mechanisms are of different importance in R7 and R8: in R7, either of the mechanisms is sufficient for color opponent processing, whereas in R8 both mechanisms are required and must act together for detectable color opponency (Schnaitmann et al. 2018). Inhibitory photoreceptor responses, synaptic contacts between photoreceptors, and expression of PxHCLB, the homolog of Drosophila HisCl1in Papilio xuthus suggest that related neuronal mechanisms underlie early color opponent processing in butterflies and flies (Takemura and Arikawa 2006; Takemura et al. 2013; Schnaitmann et al. 2018; Chen et al. 2019). Differences between Drosophila and butterflies exist in the precise site where interactions take place. In Drosophila, HisCl1-mediated photoreceptor interactions are restricted to the distal three layers m1–m3 of the medulla (Schnaitmann et al. 2018). In Papilio xuthus, the major site of PxHCLB expression is the lamina with additional neuronal expression in the medulla. Furthermore, vfs as well as svfs express the histamine receptor PxHCLB and inter-photoreceptor connectivity is more complex than in Drosophila (Chen et al. 2019).
The pale and yellow specific spectrally opponent processing mechanisms in Drosophila’s R7/R8 terminals match the opponencies that were suggested to underlie color discrimination in Lucilia (Troje 1993), and are in line with the finding that R7y/R8y are sufficient for blue–green discrimination in Drosophila (Schnaitmann et al. 2013) (see above). Together, these findings suggest that color vision in Drosophila involves UVshort/blue and UVlong/green color opponent pathways and that these pathways emerge already at the photoreceptor level. Thereby, processing in inner photoreceptor terminals very likely is the first of a series of sequential color opponent processing steps that remain to be revealed. Further color opponent processing steps in higher-order visual neurons are also suggested when the results from two-photon calcium imaging and behavioral experiments are compared. Functional imaging revealed no evidence for the integration of R1–R6 signals at the level of R7/R8 terminal responses (Schnaitmann et al. 2018). However, a contribution of R1–R6 and of their downstream neurons L1 and L2 to color discrimination has been demonstrated in a behavioral study (Schnaitmann et al. 2013; Garbers and Wachtler 2016). Therefore, the signals of R1–R6 must be integrated into the Drosophila color vision circuitry downstream of R7/R8 terminals. Combined genetic, anatomical, and behavioral studies suggest that R1–R6 signals are conveyed via L2 and/or L3 to Tm5 subtypes and Tm20, neurons that are directly downstream of R7/R8 (see below and Fig. 4). Whether these neurons indeed generate color opponent responses that are in line with indirect input from R1–R6 remains to be revealed by physiological recording.
To this date, the function of neurons downstream of R7/R8 in color vision has been addressed by combining promoter- or cell-type-specific genetic targeting with anatomical characterization, connectivity analysis, functional perturbation, and behavioral analysis (Gao et al. 2008; Karuppudurai et al. 2014; Melnattur et al. 2014). In a pioneering study, Gao et al. (2008) identified neurons that are postsynaptic to R7/R8, including several candidate neurons of the color vision circuitry. Because arthropod photoreceptors release histamine as sole neurotransmitter (Hardie 1989; Stuart 1999), the authors reasoned that direct synaptic interactions with second-order interneurons should be mediated by the inhibitory histamine-gated chloride channel Ort that is widely expressed in the lamina and medulla of the fly visual system (Witte et al. 2002; Pantazis et al. 2008). Comparison of ort-promoter activity with the stratification of axonal endings of R7/R8 revealed candidate neurons postsynaptic to R7/R8. Subsequently, synaptic connectivity was thoroughly confirmed using genetic labeling techniques (GRASP or derivatives thereof) and serial electron microscopic reconstructions (Gao et al. 2008; Takemura et al. 2013; Karuppudurai et al. 2014; Takemura et al. 2015). This resulted in the identification of the transmedulla projection neurons Tm5a/b/c, Tm9 and Tm20, and the multi-columnar amacrine neuron Dm8 (Fig. 4). Tm5a/b/c, Tm9, and Tm20 receive direct and/or indirect input from inner photoreceptors. Some of them additionally receive indirect input from R1–R6 via the lamina monopolar cells L2 and/or L3 (Takemura et al. 2013, 2015). Of these neurons Tm9 was later associated with motion vision, as it conveys information to T5 and is therefore part of the OFF-edge motion detection pathway (Shinomiya et al. 2014; Serbe et al. 2016).
Dm8 and Tm5c neurons have a central function in UV/green spectral preference. Dm8 is postsynaptic to R7, has multi-columnar organization, and expresses Ort. Individual Dm8 cells pool ‘UV input’ from 12 to 16 R7 photoreceptors of neighboring ommatidia and conveys it to all Tm5 subtypes, as well as unknown neurons (Gao et al. 2008; Karuppudurai et al. 2014; Menon et al. 2019). Blocking synaptic output of glutamatergic Dm8 results in reduced preference for UV, whereas flies with restored Ort receptor function in exclusively Dm8 (cell specific Dm8 rescue in ort mutant flies) exhibit intact spectral preference behavior (Gao et al. 2008). Knock-down of kainate glutamate receptors in postsynaptic Tm5c reduces preference to UV, suggesting that direct input from Dm8 to Tm5c is important. In line, flies with blocked synaptic transmission in Tm5c show defects in spectral preference comparable to flies with blocked synaptic transmission in Dm8 (Karuppudurai et al. 2014). Furthermore, Ort expressing Tm5c receive direct synaptic input from R8 and this input contributes to preference for green. Altogether, the R7–Dm8–Tm5c pathway is necessary and sufficient to mediate UV spectral preference against green light.
In addition to its role in UV/green spectral preference behavior, Tm5c together with other Tm cell types contributes to blue/green discrimination in a conditioning assay (Melnattur et al. 2014). Simultaneous block of Tm5a/b/c, and Tm20, but not of single cell types or combinations of them is required for complete loss of blue–green color discrimination (Melnattur et al. 2014). Therefore, Tm5a/b/c, and Tm20 are thought to represent redundant pathways of the color vision system. These neurons convey jet unknown information to visual projection neurons in the deep strata of the lobula that project to the central brain (Otsuna and Ito 2006; Lin et al. 2016; Panser et al. 2016; Wu et al. 2016). The lobula intrinsic neuron Li4 makes many synaptic contacts with almost all Tm5a/b/c and Tm20. LT11 is less connected with most of the Tms, apart from extensive Tm5a input (Lin et al. 2016). Blocking synaptic transmission in LT11, a single neuron per hemisphere that receives input from the entire visual field and therefore cannot convey retinotopic information, caused defective phototaxis specifically in the range of 410–440 nm (Otsuna et al. 2014). The same study identified another type of visual projection neuron, MC61, that projects from the medulla to the anterior optic tubercle, bypassing the lobula. Blocking its synaptic output caused a phototaxis defect in the green and UV wavelength range (Otsuna et al. 2014). Also, recent research on associative visual information processing in Drosophila revealed direct pathways from the medulla to the central brain (Vogt et al. 2014, 2016). These VPNs project to the mushroom body, a prominent brain structure involved in associative memory. Thereby, different subpopulations of VPN are required for memories of color (VPN–MB1) and brightness (VPN–MB2) (Vogt et al. 2014, 2016).
Outlook
At present, it is unknown whether or how the p–y dichotomy that has been observed in R7/R8 presynaptic terminals is retained in the pathways of the Drosophila color vision system (Schnaitmann et al. 2018). Anatomical data show that Tm5a, which is found exclusively in y columns, receives input from R7y but not R7p, and that Tm5b receives predominately input from R7p (Fig. 4c) (Karuppudurai et al. 2014; Menon et al. 2019). In contrast, Tm5c appears to pool p and y inputs (Fig. 4c). Recently, genetic and molecular methods enabled new insights into the specification of Dm8 cells. Analysis of the expression of the surface molecules of the Dpr and DIP family suggests that different flavors of Dm8 exist that likely correspond with p and y subtypes. Dpr11 is selectively expressed in R7y that contact a subpopulation of Dm8 that in turn express the Dpr11 interaction partner DIP-γ; absence of these interaction partners characterize R7p and another subpopulation of Dm8 (Carrillo et al. 2015; Tan et al. 2015; Menon et al. 2019).
What kind of color-coding physiological response types can be expected in second- and higher-order visual interneurons in Drosophila? To speculate on this question, a look at color vision in bees may provide important suggestions. Early studies on bee color vision suggested a small number of color opponencies that are likely established by deterministic wiring of the three types of photoreceptors to postsynaptic neurons. However, electrophysiological examination so far revealed a large diversity of color opponent responses with different spectral tuning (Menzel and Blakers 1976; Kien and Menzel 1977a, b; Hertel 1980; Yang et al. 2004; Paulk et al. 2008, 2009a, b). This unexpected diversity might be explained by partially random wiring of photoreceptors to postsynaptic color opponent neurons in the medulla and lobula (Vasas et al. 2019). It would also help to explain why receptive fields with a spatially antagonistic organization have not been reported so far in bees (Kien and Menzel 1977a, b; Hertel 1980). In Drosophila, it is now time to reveal the diversity of color opponent neural cell types in the medulla and lobula, the spatial layout of their receptive fields, the underlying cellular wiring, and the molecular implementation of identified computations. We expect this research to provide conceptually new insights into the neuronal basis of color vision in insects and important visual phenomena like spatial and temporal color contrast and color constancy. The recent connectomics and RNAseq data of the medulla and its cell types will fuel this research (Takemura et al. 2013, 2015; Tan et al. 2015; Konstantinides et al. 2018).
References
Arikawa K (2017) The eyes and vision of butterflies. J Physiol 595:5457–5464. https://doi.org/10.1113/JP273917
Backhaus W (1991) Color opponent coding in the visual system of the honeybee. Vision Res 31:1381–1397. https://doi.org/10.1016/0042-6989(91)90059-E
Backhaus W, Menzel R, Kreißl S (1987) Multidimensional scaling of color similarity in bees. Biol Cybern 56:293–304. https://doi.org/10.1007/BF00319510
Backhaus W, Kliegl R, Werner JS, Werner JS (1998) Color vision: perspectives from different disciplines. Walter de Gruyter, Berlin, New York
Benzer S (1967) Behavioral mutants of Drosophila isolated by countercurrent distribution. PNAS 58:1112–1119. https://doi.org/10.1073/pnas.58.3.1112
Bertholf LM (1932) The extent of the spectrum for Drosophila and the distribution of stimulative efficiency in it. Z Vergl Physiol 18:32–64. https://doi.org/10.1007/BF00338152
Borst A, Haag J, Reiff DF (2010) Fly motion vision. Annu Rev Neurosci 33:49–70. https://doi.org/10.1146/annurev-neuro-060909-153155
Borst A, Haag J, Mauss AS (2019) How fly neurons compute the direction of visual motion. J Comp Physiol A. https://doi.org/10.1007/s00359-019-01375-9
Braitenberg V (1967) Patterns of projection in the visual system of the fly. I. Retina-lamina projections. Exp Brain Res 3:271–298. https://doi.org/10.1007/bf00235589
Brandt R, Vorobyev M (1997) Metric analysis of threshold spectral sensitivity in the honeybee. Vision Res 37:425–439. https://doi.org/10.1016/S0042-6989(96)00195-2
Briscoe AD, Chittka L (2001) The evolution of color vision in insects. Annu Rev Entomol 46:471–510. https://doi.org/10.1146/annurev.ento.46.1.471
Bybee SM, Yuan F, Ramstetter MD, Llorente-Bousquets J, Reed RD, Osorio D, Briscoe AD (2012) UV photoreceptors and UV-yellow wing pigments in Heliconius butterflies allow a color signal to serve both mimicry and intraspecific communication. Am Nat 179:38–51. https://doi.org/10.1086/663192
Carrillo RA, Özkan E, Menon KP, Nagarkar-Jaiswal S, Lee P-T, Jeon M, Birnbaum ME, Bellen HJ, Garcia KC, Zinn K (2015) Control of synaptic connectivity by a network of Drosophila IgSF cell surface proteins. Cell 163:1770–1782. https://doi.org/10.1016/j.cell.2015.11.022
Chen P-J, Arikawa K, Yang E-C (2013) Diversity of the photoreceptors and spectral opponency in the compound eye of the golden birdwing. Troides aeacus formosanus. PLoS One 8:e62240. https://doi.org/10.1371/journal.pone.0062240
Chen P-J, Awata H, Matsushita A, Yang E-C, Arikawa K (2016) Extreme spectral richness in the eye of the common bluebottle butterfly, Graphium sarpedon. Front Ecol Evol 4:18. https://doi.org/10.3389/fevo.2016.00018
Chen P-J, Matsushita A, Wakakuwa M, Arikawa K (2019) Immunolocalization suggests a role of the histamine-gated chloride channel PxHCLB in spectral opponent processing in butterfly photoreceptors. J Comp Neurol 527:753–766. https://doi.org/10.1002/cne.24558
Chittka L (1992) The colour hexagon: a chromaticity diagram based on photoreceptor excitations as a generalized representation of colour opponency. J Comp Physiol A 170:533–543. https://doi.org/10.1007/BF00331193
Chittka L, Menzel R (1992) The evolutionary adaptation of flower colours and the insect pollinators’ colour vision. J Comp Physiol A 171:171–181. https://doi.org/10.1007/BF00188925
Chittka L, Beier W, Hertel H, Steinmann E, Menzel R (1992) Opponent colour coding is a universal strategy to evaluate the photoreceptor inputs in Hymenoptera. J Comp Physiol A 170:545–563. https://doi.org/10.1007/BF00199332
Chittka L, Faruq S, Skorupski P, Werner A (2014) Colour constancy in insects. J Comp Physiol A 200:435–448. https://doi.org/10.1007/s00359-014-0897-z
Chou WH, Hall KJ, Wilson DB, Wideman CL, Townson SM, Chadwell LV, Britt SG (1996) Identification of a novel Drosophila opsin reveals specific patterning of the R7 and R8 photoreceptor cells. Neuron 17:1101–1115. https://doi.org/10.1016/s0896-6273(00)80243-3
Chou WH, Huber A, Bentrop J, Schulz S, Schwab K, Chadwell LV, Paulsen R, Britt SG (1999) Patterning of the R7 and R8 photoreceptor cells of Drosophila: evidence for induced and default cell-fate specification. Development 126:607–616
Cuthill IC, Allen WL, Arbuckle K, Caspers B, Chaplin G, Hauber ME, Hill GE, Jablonski NG, Jiggins CD, Kelber A, Mappes J, Marshall J, Merrill R, Osorio D, Prum R, Roberts NW, Roulin A, Rowland HM, Sherratt TN, Skelhorn J, Speed MP, Stevens M, Stoddard MC, Stuart-Fox D, Talas L, Tibbetts E, Caro T (2017) The biology of color. Science 357:eaan0221. https://doi.org/10.1126/science.aan0221
Dacey DM, Packer OS (2003) Colour coding in the primate retina: diverse cell types and cone-specific circuitry. Curr Opin in Neurobiol 13:421–427. https://doi.org/10.1016/S0959-4388(03)00103-X
Demb JB, Singer JH (2015) Functional circuitry of the retina. Annu Rev Vis Sci 1:263–289. https://doi.org/10.1146/annurev-vision-082114-035334
Dickinson MH (2014) Death valley, Drosophila, and the Devonian toolkit. Annu Rev Entomol 59:51–72. https://doi.org/10.1146/annurev-ento-011613-162041
Dyer AG, Boyd-Gerny S, McLoughlin S, Rosa MGP, Simonov V, Wong BBM (2012) Parallel evolution of angiosperm colour signals: common evolutionary pressures linked to hymenopteran vision. Proc Biol Sci 279:3606–3615. https://doi.org/10.1098/rspb.2012.0827
Fingerman M, Brown FA (1952) A “Purkinje shift” in insect vision. Science 116:171–172. https://doi.org/10.1126/science.116.3007.171
Fischbach KF (1979) Simultaneous and successive colour contrast expressed in “slow” phototactic behaviour of walking Drosophila melanogaster. J Comp Physiol 130:161–171. https://doi.org/10.1007/BF00611050
Fischbach K, Dittrich A (1989) The optic lobe of Drosophila melanogaster. 1. A Golgi analysis of wildtype structure. Cell Tissue Res 258:441–475. https://doi.org/10.1007/BF00218858
Franceschini N, Kirschfeld K, Minke B (1981) Fluorescence of photoreceptor cells observed in vivo. Science 213:1264–1267. https://doi.org/10.1126/science.7268434
Gao S, Takemura S, Ting C-Y, Huang S, Lu Z, Luan H, Rister J, Thum AS, Yang M, Hong S-T, Wang JW, Odenwald WF, White BH, Meinertzhagen IA, Lee C-H (2008) The neural substrate of spectral preference in Drosophila. Neuron 60:328–342. https://doi.org/10.1016/j.neuron.2008.08.010
Garbers C, Wachtler T (2016) Wavelength discrimination in Drosophila suggests a role of Rhodopsin 1 in color vision. PLoS One 11:e0155728. https://doi.org/10.1371/journal.pone.0155728
Garcia JE, Girard MB, Kasumovic M, Petersen P, Wilksch PA, Dyer AG (2015) Differentiating biological colours with few and many sensors: spectral reconstruction with RGB and hyperspectral cameras. PLoS One 10:e0125817. https://doi.org/10.1371/journal.pone.0125817
Gegenfurtner KR (2003) Cortical mechanisms of colour vision. Nat Rev Neurosci 4:563–572. https://doi.org/10.1038/nrn1138
Gegenfurtner KR, Kiper DC (2003) Color vision. Annu Rev Neurosci 26:181–206. https://doi.org/10.1146/annurev.neuro.26.041002.131116
Giurfa M (2004) Conditioning procedure and color discrimination in the honeybee Apis mellifera. Naturwissenschaften 91:228–231. https://doi.org/10.1007/s00114-004-0530-z
Giurfa M, Vorobyev M (1998) The angular range of achromatic target detection by honey bees. J Comp Physiol A 183:101–110. https://doi.org/10.1007/s003590050238
Giurfa M, Vorobyev M, Kevan P, Menzel R (1996) Detection of coloured stimuli by honeybees: minimum visual angles and receptor specific contrasts. J Comp Physiol A 178:699–709. https://doi.org/10.1007/BF00227381
Giurfa M, Vorobyev M, Brandt R, Posner B, Menzel R (1997) Discrimination of coloured stimuli by honeybees: alternative use of achromatic and chromatic signals. J Comp Physiol A 180:235–243. https://doi.org/10.1007/s003590050044
Guntur AR, Gou B, Gu P, He R, Stern U, Xiang Y, Yang C-H (2017) H2O2-sensitive isoforms of Drosophila melanogaster TRPA1 act in bitter-sensing gustatory neurons to promote avoidance of UV during egg-laying. Genetics 205:749–759. https://doi.org/10.1534/genetics.116.195172
Hardie RC (1989) A histamine-activated chloride channel involved in neurotransmission at a photoreceptor synapse. Nature 339:704–706. https://doi.org/10.1038/339704a0
Hardie RC (2001) Phototransduction in Drosophila melanogaster. J Exp Biol 204:3403–3409
Hardie RC, Juusola M (2015) Phototransduction in Drosophila. Curr Opin in Neurobiol 34:37–45. https://doi.org/10.1016/j.conb.2015.01.008
Harris WA, Stark JA, Walker WS (1976) Genetic dissection of the photoreceptor system in the compound eye of Drosophila melanogaster. J Physiol 256:415–439
Hawkes MF, Duffy E, Joag R, Skeats A, Radwan J, Wedell N, Sharma MD, Hosken DJ, Troscianko J (2019) Sexual selection drives the evolution of male wing interference patterns. Proc Biol Sci 286:20182850. https://doi.org/10.1098/rspb.2018.2850
Heisenberg M, Buchner E (1977) The rôle of retinula cell types in visual behavior of Drosophila melanogaster. J Comp Physiol 117:127–162. https://doi.org/10.1007/BF00612784
Hempel de Ibarra N, Vorobyev M, Menzel R (2014) Mechanisms, functions and ecology of colour vision in the honeybee. J Comp Physiol Neuroethol Sens Neural Behav Physiol 200:411–433. https://doi.org/10.1007/s00359-014-0915-1
Hering E (1878) Zur Lehre vom Lichtsinne, 2, unveränderte. Gerold, Wien
Hernández de Salomon CH, Spatz HC (1983) Colour vision in Drosophila melanogaster: wavelength discrimination. J Comp Physiol 150:31–37. https://doi.org/10.1007/BF00605285
Hertel H (1980) Chromatic properties of identified interneurons in the optic lobes of the bee. J Comp Physiol 137:215–231. https://doi.org/10.1007/BF00657117
Horridge GA, Marčelja L, Jahnke R, Matič T (1983) Single electrode studies on the retina of the butterfly Papilio. J Comp Physiol 150:271–294. https://doi.org/10.1007/BF00605018
Hu KG, Stark WS (1977) Specific receptor input into spectral preference in Drosophila. J Comp Physiol 121:241–252. https://doi.org/10.1007/BF00609614
Jacob KG, Willmund R, Folkers E, Fischbach KF, Spatz HC (1977) T-maze phototaxis of Drosophila melanogaster and several mutants in the visual systems. J Comp Physiol 116:209–225. https://doi.org/10.1007/BF00605403
Jacobs GH (2008) Primate color vision: a comparative perspective. Vis Neurosci 25:619–633. https://doi.org/10.1017/S0952523808080760
Jacobs GH (2014) The discovery of spectral opponency in visual systems and its impact on understanding the neurobiology of color vision. J Hist Neurosci 23:287–314. https://doi.org/10.1080/0964704X.2014.896662
Johnston RJ (2013) Lessons about terminal differentiation from the specification of color-detecting photoreceptors in the Drosophila retina. Ann N Y Acad Sci 1293:33–44. https://doi.org/10.1111/nyas.12178
Kaiser W, Seidl R, Vollmar J (1977) The participation of all three colour receptors in the phototactic behaviour of fixed walking honeybees. J Comp Physiol 122:27–44. https://doi.org/10.1007/BF00611246
Karuppudurai T, Lin T-Y, Ting C-Y, Pursley R, Melnattur KV, Diao F, White BH, Macpherson LJ, Gallio M, Pohida T, Lee C-H (2014) A hard-wired glutamatergic circuit pools and relays UV signals to mediate spectral preference in Drosophila. Neuron 81:603–615. https://doi.org/10.1016/j.neuron.2013.12.010
Katayama N, Abbott JK, Kjærandsen J, Takahashi Y, Svensson EI (2014) Sexual selection on wing interference patterns in Drosophila melanogaster. PNAS 111:15144–15148. https://doi.org/10.1073/pnas.1407595111
Kelber A (2001) Receptor based models for spontaneous colour choices in flies and butterflies. Entomol Exp Appl 99:231–244. https://doi.org/10.1046/j.1570-7458.2001.00822.x
Kelber A, Osorio D (2010) From spectral information to animal colour vision: experiments and concepts. Proc R Soc B 277:1617–1625. https://doi.org/10.1098/rspb.2009.2118
Kelber A, Vorobyev M, Osorio D (2003) Animal colour vision—behavioural tests and physiological concepts. Biol Rev 78:81–118. https://doi.org/10.1017/S1464793102005985
Kien J, Menzel R (1977a) Chromatic properties of interneurons in the optic lobes of the bee. J Comp Physiol 113:17–34. https://doi.org/10.1007/BF00610451
Kien J, Menzel R (1977b) Chromatic properties of interneurons in the optic lobes of the bee. J Comp Physiol 113:35–53. https://doi.org/10.1007/BF00610452
Kirschfeld K (1973) Neural superposition eye. Fortschr Zool 21:229–257
Koenig J, Merriam JR (1977) Autosomal ERG mutants. Dros Inf Serv 52:50–51
Konstantinides N, Kapuralin K, Fadil C, Barboza L, Satija R, Desplan C (2018) Phenotypic convergence: distinct transcription factors regulate common terminal features. Cell 174:622–635. https://doi.org/10.1016/j.cell.2018.05.021
Koshitaka H, Kinoshita M, Vorobyev M, Arikawa K (2008) Tetrachromacy in a butterfly that has eight varieties of spectral receptors. Proc R Soc B 275:947–954. https://doi.org/10.1098/rspb.2007.1614
Kronforst MR, Young LG, Kapan DD, McNeely C, O’Neill RJ, Gilbert LE (2006) Linkage of butterfly mate preference and wing color preference cue at the genomic location of wingless. PNAS 103:6575–6580. https://doi.org/10.1073/pnas.0509685103
Langen M, Agi E, Altschuler DJ, Wu LF, Altschuler SJ, Hiesinger PR (2015) The developmental rules of neural superposition in Drosophila. Cell 162:120–133. https://doi.org/10.1016/j.cell.2015.05.055
Lazopulo S, Lazopulo A, Baker JD, Syed S (2019) Daytime colour preference in Drosophila depends on the circadian clock and TRP channels. Nature 574:108–111. https://doi.org/10.1038/s41586-019-1571-y
Lebhardt F, Desplan C (2017) Retinal perception and ecological significance of color vision in insects. Curr Opin Insect Sci 24:75–83. https://doi.org/10.1016/j.cois.2017.09.007
Lin T-Y, Luo J, Shinomiya K, Ting C-Y, Lu Z, Meinertzhagen IA, Lee C-H (2016) Mapping chromatic pathways in the Drosophila visual system. J Comp Neurol 524:213–227. https://doi.org/10.1002/cne.23857
Lunau K (2014) Visual ecology of flies with particular reference to colour vision and colour preferences. J Comp Physiol A 200:497–512. https://doi.org/10.1007/s00359-014-0895-1
Lunau K, Wacht S (1994) Optical releasers of the innate proboscis extension in the hoverfly Eristalis tenax L. (Syrphidae, Diptera). J Comp Physiol A 174:575–579. https://doi.org/10.1007/BF00217378
Mathejczyk TF, Wernet MF (2017) Sensing polarized light in insects. Oxf Res Encyclopedia Neurosci. https://doi.org/10.1093/acrefore/9780190264086.013.109
Matić T (1983) Electrical inhibition in the retina of the butterfly Papilio. J Comp Physiol 152:169–182. https://doi.org/10.1007/BF00611182
Mazzoni EO, Celik A, Wernet MF, Vasiliauskas D, Johnston RJ, Cook TA, Pichaud F, Desplan C (2008) Iroquois Complex genes induce co-expression of rhodopsins in Drosophila. PLoS Biol 6:e97. https://doi.org/10.1371/journal.pbio.0060097
Meinertzhagen IA, O’Neil SD (1991) Synaptic organization of columnar elements in the lamina of the wild type in Drosophila melanogaster. J Comp Neurol 305:232–263. https://doi.org/10.1002/cne.903050206
Melin AD, Kline DW, Hickey CM, Fedigan LM (2013) Food search through the eyes of a monkey: a functional substitution approach for assessing the ecology of primate color vision. Vision Res 86:87–96. https://doi.org/10.1016/j.visres.2013.04.013
Melnattur KV, Pursley R, Lin T-Y, Ting C-Y, Smith PD, Pohida T, Lee C-H (2014) Multiple redundant medulla projection neurons mediate color vision in Drosophila. J Neurogenet 28:374–388. https://doi.org/10.3109/01677063.2014.891590
Menne D, Spatz H-C (1977) Colour vision in Drosophila melanogaster. J Comp Physiol 114:301–312. https://doi.org/10.1007/BF00657325
Menon KP, Kulkarni V, Takemura S-Y, Anaya M, Zinn K (2019) Interactions between Dpr11 and DIP-γ control selection of amacrine neurons in Drosophila color vision circuits. eLife 8:e48935. https://doi.org/10.7554/eLife.48935
Menzel R, Backhaus W (1991) Colour vision in insects. In: Gouras P (ed) Vision and visual dysfunction. The perception of colour. MacMillan Press, London, pp 262–288
Menzel R, Blakers M (1976) Colour receptors in the bee eye—morphology and spectral sensitivity. J Comp Physiol 108:11–13. https://doi.org/10.1007/BF00625437
Menzel R, Greggers U (1985) Natural phototaxis and its relationship to colour vision in honeybees. J Comp Physiol A 157:311–321. https://doi.org/10.1007/BF00618121
Mikeladze-Dvali T, Desplan C, Pistillo D (2005) Flipping coins in the fly retina. Curr Top Dev Biol 69:1–15. https://doi.org/10.1016/S0070-2153(05)69001-1
Möller R (2002) Insects could exploit UV-green contrast for landmark navigation. J Theor Biol 214:619–631. https://doi.org/10.1006/jtbi.2001.2484
Mollon JD (1999) Color vision: opsins and options. PNAS 96:4743–4745. https://doi.org/10.1073/pnas.96.9.4743
Mote MI, Goldsmith TH (1970) Spectral sensitivities of color receptors in the compound eye of the cockroach Periplaneta. J Exp Zool 173:137–145. https://doi.org/10.1002/jez.1401730203
Neumeyer C (1980) Simultaneous color contrast in the honeybee. J Comp Physiol 139:165–176. https://doi.org/10.1007/BF00657079
Ng L, Garcia JE, Dyer AG (2018) Why colour is complex: evidence that bees perceive neither brightness nor green contrast in colour signal processing. FACETS. https://doi.org/10.1139/facets-2017-0116
Niggebrügge C, Leboulle G, Menzel R, Komischke B, de Ibarra NH (2009) Fast learning but coarse discrimination of colours in restrained honeybees. J Exp Biol 212:1344–1350
Niven JE, Laughlin SB (2008) Energy limitation as a selective pressure on the evolution of sensory systems. J Exp Biol 211:1792–1804. https://doi.org/10.1242/jeb.017574
O’tousa JE, Leonard DS, Pak WL (1989) Morphological defects in oraJK84 photoreceptors caused by mutation in R1-6 opsin gene of Drosophila. J Neurogenet 6:41–52. https://doi.org/10.3109/01677068909107099
Osorio D, Cuthill I (2015) Camouflage and perceptual organization in the animal kingdom. In: Wagemans J (ed) The Oxford handbook of perceptual organization. Oxford library of psychology. Oxford University Press, Oxford, pp 843–862. https://doi.org/10.1093/oxfordhb/9780199686858.013.044
Osorio D, Vorobyev M (2005) Photoreceptor spectral sensitivities in terrestrial animals: adaptations for luminance and colour vision. Proc R Soc B 272:1745–1752. https://doi.org/10.1098/rspb.2005.3156
Osorio D, Vorobyev M (2008) A review of the evolution of animal colour vision and visual communication signals. Vision Res 48:2042–2051. https://doi.org/10.1016/j.visres.2008.06.018
Otsuna H, Ito K (2006) Systematic analysis of the visual projection neurons of Drosophila melanogaster. I. Lobula-specific pathways. J Comp Neurol 497:928–958. https://doi.org/10.1002/cne.21015
Otsuna H, Shinomiya K, Ito K (2014) Parallel neural pathways in higher visual centers of the Drosophila brain that mediate wavelength-specific behavior. Front Neural Circuits 8:8. https://doi.org/10.3389/fncir.2014.00008
Panser K, Tirian L, Schulze F, Villalba S, Jefferis GSXE, Bühler K, Straw AD (2016) Automatic segmentation of Drosophila neural compartments using GAL4 expression data reveals novel visual pathways. Curr Biol 26:1943–1954. https://doi.org/10.1016/j.cub.2016.05.052
Pantazis A, Segaran A, Liu C-H, Nikolaev A, Rister J, Thum AS, Roeder T, Semenov E, Juusola M, Hardie RC (2008) Distinct roles for two histamine receptors (hclA and hclB) at the Drosophila photoreceptor synapse. J Neurosci 28:7250–7259. https://doi.org/10.1523/JNEUROSCI.1654-08.2008
Paulk AC, Phillips-Portillo J, Dacks AM, Fellous JM, Gronenberg W (2008) The processing of color, motion, and stimulus timing are anatomically segregated in the bumblebee brain. J Neurosci 28:6319–6332. https://doi.org/10.1523/JNEUROSCI.1196-08.2008
Paulk AC, Dacks AM, Gronenberg W (2009a) Color processing in the medulla of the bumblebee (Apidae: Bombus impatiens). J Comp Neurol 513:441–456. https://doi.org/10.1002/cne.21993
Paulk AC, Dacks AM, Phillips-Portillo J, Fellous JM, Gronenberg W (2009b) Visual processing in the central bee brain. J Neurosci 29:9987–9999. https://doi.org/10.1523/JNEUROSCI.1325-09.2009
Perry M, Kinoshita M, Saldi G, Huo L, Arikawa K, Desplan C (2016) Molecular logic behind the three-way stochastic choices that expand butterfly colour vision. Nature 535:280–284. https://doi.org/10.1038/nature18616
Poulton EB (1890) The colours of animals: their meaning and use, especially considered in the case of insects. Kegan Paul, Trench, Trübner, London
Regan BC, Julliot C, Simmen B, Viénot F, Charles-Dominique P, Mollon JD (2001) Fruits, foliage and the evolution of primate colour vision. Philos Trans R Soc Lond B 356:229–283. https://doi.org/10.1098/rstb.2000.0773
Ribi WA (1981) The first optic ganglion of the bee. IV. Synaptic fine structure and connectivity patterns of receptor cell axons and first order interneurones. Cell Tissue Res 215:443–464. https://doi.org/10.1007/bf00233522
Rushton WAH (1972) Review lecture. Pigments and signals in colour vision. J Physiol 220:1–31
Salcedo E, Huber A, Henrich S, Chadwell LV, Chou W-H, Paulsen R, Britt SG (1999) Blue- and green-absorbing visual pigments of Drosophila: ectopic expression and physiological characterization of the R8 photoreceptor cell-specific Rh5 and Rh6 rhodopsins. J Neurosci 19:10716–10726. https://doi.org/10.1523/JNEUROSCI.19-24-10716.1999
Schnaitmann C, Vogt K, Triphan T, Tanimoto H (2010) Appetitive and aversive visual learning in freely moving Drosophila. Front Behav Neurosci 4:10. https://doi.org/10.3389/fnbeh.2010.00010
Schnaitmann C, Garbers C, Wachtler T, Tanimoto H (2013) Color discrimination with broadband photoreceptors. Curr Biol 23:2375–2382. https://doi.org/10.1016/j.cub.2013.10.037
Schnaitmann C, Haikala V, Abraham E, Oberhauser V, Thestrup T, Griesbeck O, Reiff DF (2018) Color processing in the early visual system of Drosophila. Cell 172:318–330. https://doi.org/10.1016/j.cell.2017.12.018
Schümperli RA (1973) Evidence for colour vision in Drosophila melanogaster through spontaneous phototactic choice behaviour. J Comp Physiol 86:77–94. https://doi.org/10.1007/BF00694480
Serbe E, Meier M, Leonhardt A, Borst A (2016) Comprehensive characterization of the major presynaptic elements to the Drosophila OFF motion detector. Neuron 89:829–841. https://doi.org/10.1016/j.neuron.2016.01.006
Shevtsova E, Hansson C, Janzen DH, Kjærandsen J (2011) Stable structural color patterns displayed on transparent insect wings. PNAS 108:668–673. https://doi.org/10.1073/pnas.1017393108
Shinomiya K, Karuppudurai T, Lin T-Y, Lu Z, Lee C-H, Meinertzhagen IA (2014) Candidate neural substrates for off-edge motion detection in Drosophila. Curr Biol 24:1062–1070. https://doi.org/10.1016/j.cub.2014.03.051
Shrestha M, Lunau K, Dorin A, Schulze B, Bischoff M, Burd M, Dyer AG (2016) Floral colours in a world without birds and bees: the plants of Macquarie Island. Plant Biol 18:842–850. https://doi.org/10.1111/plb.12456
Shrestha M, Dyer AG, Garcia JE, Burd M (2019) Floral colour structure in two Australian herbaceous communities: it depends on who is looking. Ann Bot 124:221–232. https://doi.org/10.1093/aob/mcz043
Skorupski P, Döring TF, Chittka L (2007) Photoreceptor spectral sensitivity in island and mainland populations of the bumblebee, Bombus terrestris. J Comp Physiol A 193:485–494. https://doi.org/10.1007/s00359-006-0206-6
Solomon SG, Lennie P (2007) The machinery of colour vision. Nat Rev Neurosci 8:276–286. https://doi.org/10.1038/nrn2094
Stuart AE (1999) From fruit flies to barnacles, histamine is the neurotransmitter of arthropod photoreceptors. Neuron 22:431–433. https://doi.org/10.1016/s0896-6273(00)80699-6
Swihart SL (1972) The neural basis of colour vision in the butterfly, Heliconius erato. J Insect Physiol 18:1015–1025. https://doi.org/10.1016/0022-1910(72)90038-8
Takemura S-Y, Arikawa K (2006) Ommatidial type-specific interphotoreceptor connections in the lamina of the swallowtail butterfly, Papilio xuthus. J Comp Neurol 494:663–672. https://doi.org/10.1002/cne.20830
Takemura S-Y, Kinoshita M, Arikawa K (2005) Photoreceptor projection reveals heterogeneity of lamina cartridges in the visual system of the Japanese yellow swallowtail butterfly, Papilio xuthus. J Comp Neurol 483:341–350. https://doi.org/10.1002/cne.20446
Takemura SY, Lu Z, Meinertzhagen IA (2008) Synaptic circuits of the Drosophila optic lobe: the input terminals to the medulla. J Comp Neurol 509:493–513. https://doi.org/10.1002/cne.21757
Takemura S, Bharioke A, Lu Z, Nern A, Vitaladevuni S, Rivlin PK, Katz WT, Olbris DJ, Plaza SM, Winston P, Zhao T, Horne JA, Fetter RD, Takemura S, Blazek K, Chang L-A, Ogundeyi O, Saunders MA, Shapiro V, Sigmund C, Rubin GM, Scheffer LK, Meinertzhagen IA, Chklovskii DB (2013) A visual motion detection circuit suggested by Drosophila connectomics. Nature 500:175–181. https://doi.org/10.1038/nature12450
Takemura S, Xu CS, Lu Z, Rivlin PK, Parag T, Olbris DJ, Plaza S, Zhao T, Katz WT, Umayam L, Weaver C, Hess HF, Horne JA, Nunez-Iglesias J, Aniceto R, Chang L-A, Lauchie S, Nasca A, Ogundeyi O, Sigmund C, Takemura S, Tran J, Langille C, Lacheur KL, McLin S, Shinomiya A, Chklovskii DB, Meinertzhagen IA, Scheffer LK (2015) Synaptic circuits and their variations within different columns in the visual system of Drosophila. PNAS 112:13711–13716. https://doi.org/10.1073/pnas.1509820112
Tan L, Zhang KX, Pecot MY, Nagarkar-Jaiswal S, Lee P-T, Takemura S-Y, McEwen JM, Nern A, Xu S, Tadros W, Chen Z, Zinn K, Bellen HJ, Morey M, Zipursky SL (2015) Ig superfamily ligand and receptor pairs expressed in synaptic partners in Drosophila. Cell 163:1756–1769. https://doi.org/10.1016/j.cell.2015.11.021
Tang S, Guo A (2001) Choice behavior of Drosophila facing contradictory visual cues. Science 294:1543–1547. https://doi.org/10.1126/science.1058237
Troje N (1993) Spectral categories in the learning behaviour of blowflies. Z Naturforsch C 48:96. https://doi.org/10.1515/znc-1993-1-218
Trujillo-Cenóz O, Melamed J (1966) Compound eye of dipterans: anatomical basis for integration—an electron microscope study. J Ultrastruct Res 16:395–398
Tuthill JC, Nern A, Holtz SL, Rubin GM, Reiser MB (2013) Contributions of the 12 neuron classes in the fly lamina to motion vision. Neuron 79:128–140. https://doi.org/10.1016/j.neuron.2013.05.024
van Hateren JH (1993) Spatial, temporal and spectral pre-processing for colour vision. Proc Biol Sci 251:61–68. https://doi.org/10.1098/rspb.1993.0009
Vasas V, Peng F, MaBouDi H, Chittka L (2019) Randomly weighted receptor inputs can explain the large diversity of colour-coding neurons in the bee visual system. Sci Rep 9:8330. https://doi.org/10.1038/s41598-019-44375-0
Vogt K, Schnaitmann C, Dylla KV, Knapek S, Aso Y, Rubin GM, Tanimoto H (2014) Shared mushroom body circuits underlie visual and olfactory memories in Drosophila. eLife 3:e02395. https://doi.org/10.7554/eLife.02395
Vogt K, Aso Y, Hige T, Knapek S, Ichinose T, Friedrich AB, Turner GC, Rubin GM, Tanimoto H (2016) Direct neural pathways convey distinct visual information to Drosophila mushroom bodies. eLife 5:e14009. https://doi.org/10.7554/eLife.14009
von Frisch K (1914) Der Farbensinn und Formensinn der Biene. Zool Jb Physiol 35:1–188
von Helversen O (1972) Zur spektralen unterschiedsempfindlichkeit der Honigbiene. J Comp Physiol 80:439–472
Vorobyev M, Brandt R, Peitsch D, Laughlin SB, Menzel R (2001) Colour thresholds and receptor noise: behaviour and physiology compared. Vision Res 41:639–653. https://doi.org/10.1016/S0042-6989(00)00288-1
Wakakuwa M, Kurasawa M, Giurfa M, Arikawa K (2005) Spectral heterogeneity of honeybee ommatidia. Naturwissenschaften 92:464–467
Wardill TJ, List O, Li X, Dongre S, McCulloch M, Ting C-Y, O’Kane CJ, Tang S, Lee C-H, Hardie RC, Juusola M (2012) Multiple spectral inputs improve motion discrimination in the Drosophila visual system. Science 336:925–931. https://doi.org/10.1126/science.1215317
Weiss MR (1991) Floral colour changes as cues for pollinators. Nature 354:227–229. https://doi.org/10.1038/354227a0
Wernet MF, Mazzoni EO, Çelik A, Duncan DM, Duncan I, Desplan C (2006) Stochastic spineless expression creates the retinal mosaic for colour vision. Nature 440:174–180. https://doi.org/10.1038/nature04615
Witte I, Kreienkamp H-J, Gewecke M, Roeder T (2002) Putative histamine-gated chloride channel subunits of the insect visual system and thoracic ganglion. J Neurochem 83:504–514. https://doi.org/10.1046/j.1471-4159.2002.01076.x
Wu M, Nern A, Williamson WR, Morimoto MM, Reiser MB, Card GM, Rubin GM (2016) Visual projection neurons in the Drosophila lobula link feature detection to distinct behavioral programs. eLife 5:e21022. https://doi.org/10.7554/eLife.21022
Yamaguchi S, Wolf R, Desplan C, Heisenberg M (2008) Motion vision is independent of color in Drosophila. PNAS 105:4910–4915. https://doi.org/10.1073/pnas.0711484105
Yamaguchi S, Desplan C, Heisenberg M (2010) Contribution of photoreceptor subtypes to spectral wavelength preference in Drosophila. PNAS 107:5634–5639. https://doi.org/10.1073/pnas.0809398107
Yang E-C, Lin HC, Hung YS (2004) Patterns of chromatic information processing in the lobula of the honeybee, Apis mellifera L. J Insect Physiol 50:913–925. https://doi.org/10.1016/j.jinsphys.2004.06.010
Yilmaz A, Dyer AG, Rössler W, Spaethe J (2017) Innate colour preference, individual learning and memory retention in the ant Camponotus blandus. J Exp Biol 220:3315–3326. https://doi.org/10.1242/jeb.158501
Zhu EY, Guntur AR, He R, Stern U, Yang C-H (2014) Egg-laying demand induces aversion of UV light in Drosophila females. Curr Biol 24:2797–2804. https://doi.org/10.1016/j.cub.2014.09.076
Acknowledgements
Open Access funding provided by Projekt DEAL. This work was supported by a DFG grant to D.F. Reiff (DFG—RE 3089/1-1) and the state of Baden-Württemberg.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schnaitmann, C., Pagni, M. & Reiff, D.F. Color vision in insects: insights from Drosophila. J Comp Physiol A 206, 183–198 (2020). https://doi.org/10.1007/s00359-019-01397-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-019-01397-3