Abstract
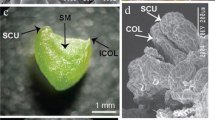
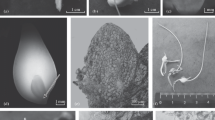
Freesia hybrida is an important worldwide cut flower, especially in America and Europe. For efficient regeneration of this flower from young inflorescence and rachillae in tetraploid, we developed a simple in vitro micropropagation protocol. Explants of Freesia hybrida can regenerate plantlets through somatic embryogenesis via two kinds of pathways, that is, directly from the epidermal cells or indirectly from an embryonic callus, depending on the exogenous plant growth regulators (PGRs) used in the culture media. In direct embryogenesis, when the explants were cultured on Murashige and Skoog (MS) medium supplemented with 11.43 μM indole acetic acid (IAA) and 4.44 μM 6-benzylaminopurine (6-BA), the induction rate was 84% for young inflorescence and 100% for rachillae. After the multishoots were subcultured on the rooting MS medium containing 1.08 μM α-naphthalene acetic acid (NAA), the rooting rate was close to 100%. In indirect embryogenesis, embryonic calluses were formed when the culture medium contained 22.22 μM 6-BA and 4.52 μM 2,4-dichlorophenoxy acetic acid (2,4-D), and the induction rate was 92.4% for young inflorescence and 100% for rachillae. After the embryonic calluses were transferred to the medium supplemented with 11.43 μM IAA and 13.33 μM 6-BA, they could develop into plantlets with roots. In assessing the two regeneration pathways in terms of genetic and epigenetic fidelity of the regenerants, two kinds of molecular markers [amplified fragment length polymorphism (AFLP) and methylation-sensitive amplified polymorphism (MSAP)] were employed. The AFLP analysis used 20 primer pairs that yielded 916 scorable bands among the donor plant and 11 regenerants from direct embryogenesis, of which 8 (0.87%) were polymorphic. The regenerants from indirect embryogenesis had 1075 clear bands of which 3 (0.27%) were polymorphic scorable bands from 18 primer pairs. Moreover, the variant band patterns included two types, that is, loss-of-original and gain-of-novel bands. MSAP analysis revealed that tissue culturing of the flower induced DNA cytosine methylation alterations in both CG and CNG levels and patterns compared with the donor plant. The variation rate was 1.1 and 1.3% for the direct and indirect embryogenesis pathways, respectively. The findings show that tissue culture of flowering plants is a form of stress which can induce some heritable epigenetic variations and should be considered in future long-term genotype preservation programs of Freesia hybrida.




Similar content being viewed by others
References
Arnold S, Sabala I, Bozhkov P, Dyachok J, Filonova L (2002) Developmental pathways of somatic embryogenesis. Plant Cell Tissue Organ Cult 69:233–249
Ashikawa I (2001) Surveying CpG methylation at 5′-CCGG in the genomes of rice cultivars. Plant Mol Biol 45:31–39
Bairu MW, Fennell CW, van Staden J (2006) The effect of plant growth regulators on somaclonal variation in Cavendish banana (Musa AAA cv’.Zelig’). Sci Hortic 108:347–351
Baurens FC, Bonnot F, Bienvenu D, Causse S, Legavre T (2003) Using SD-AFLP and MSAP to assess CCGG methylation in the banana genome. Plant Mol Biol Rep 21:339–348
Bayliss MW (1980) Chromosomal variation in tissue culture. Int Rev Cytol Suppl 11A:113–144
Bohorova NE, Luna B, Briton RM, Huerta LD, Hoisington DA (1995) Regeneration potential of tropical, and subtropical, midaltitude, and highland maize inbreds. Maydica 40:275–281
Bregitzer P, Campbell RD, Wu Y (1995) Plant regeneration from barley callus: effects of 2,4-dichlorphenoxy acetic acid and phenylacetic acid. Plant Cell Tissue Organ Cult 43:229–235
Brown PTH (1989) DNA methylation in plants and its role in tissue culture. Genome 31(2):717–729
Brown PTH, Kyozuka J, Sukekiyo Y, Kimura Y, Shimamoto K, Lörz H (1990) Molecular changes in protoplast-derived rice plants. Mol Gen Genet 223:324–328
Brown PTH, Gobel E, Lorz H (1991) RFLP analysis of Zea mays callus cultures and their regenerated plants. Theor Appl Genet 81:227–232
Brown DCW, Finstad KI, Watson EM (1995) Somatic embryogenesis in herbaceous species. In: Thorpe TA (ed) In vitro embryogenesis in plants. Kluwer, Dordrecht, The Netherlands, pp 345–415
Carvalho CHS, Bohorova N, Bordallo PN, Abreu LL, Valicente FH, Bressan W, Paiva E (1997) Type II callus production and plant regeneration in tropical maize genotypes. Plant Cell Rep 17:73–76
Cecchini E, Natali L, Cavallini A, Durante M (1992) DNA variations in regenerated plants of pea (Pisum sativum L.). Theor Appl Genet 84:874–879
Cervera MT, Ruiz-Garcia L, Martinez-Zapater JM (2002) Analysis of DNA methylation in Arabidopsis thaliana based on methylation sensitive AFLP markers. Mol Genet Genomics 268:543–552
Chakrabarty D, Yu KW, Paek KY (2003) Detection of DNA methylation changes during somatic embryogenesis of Siberian ginseng (Eleuterococcus senticosus). Plant Sci 165:61–68
Creissen SS, Karp A (1985) Karyotypic changes in potato plants regenerated from protoplasts. Plant Cell Tissue Organ Cult 4:171–182
Damasco OP, Smith MK, Adkins SW, Godwin ID (1998) Use of SCAR based marker for early detection of dwarf off-types in micropropagated ‘Cavendish’ bananas. Acta Hort 461:157–164
Dunstan DI, Tautorus TE, Thorpe TA (1995) Somatic embryogenesis in woody plants. In: Thorpe TA (ed) In vitro embryogenesis in plants. Kluwer, Dordrecht, The Netherlands, pp 471–541
Evans DA, Sharp WR, Medina-Filho HP (1984) Somaclonal and gametoclonal variation. Am J Bot 77:759–774
Feil R (2006) Environmental and nutritional effects on the epigenetic regulation of genes. Mutat Res 600:46–57
Gostimsky SA, Kokaeva ZG, Konovalov FA (2005) Studying plant genome variation using molecular markers. Russ J Genet 41:378–388
Gruenbaum Y, Naveh-Many T, Cedar A, Razin A (1981) Sequence specificity of methylation in higher plant DNA. Nature 292:860–862
Guo WL, Gong L, Ding ZF, Li YD, Li FX, Zhao SP, Liu B (2006) Genomic instability in phenotypically normal regenerants of medicinal plant Codonopsis lanceolata Benth. et Hook. f., as revealed by ISSR and RAPD markers. Plant Cell Rep 25:896–906
Guo WL, Wu R, Zhang YF, Liu XM, Wang HY, Gong L, Zhang ZH, Liu B (2007) Tissue culture-induced locus-specific alteration in DNA methylation and its correlation with genetic variation in Codonopsis lanceolata Benth. et Hook. f. Plant Cell Rep 26:1297–1307
Jain SM (1998) Plant biotechnology and mutagenesis for sustainable crop improvement In: Behl RK, Singh DK, Lodhi GP (eds) Crop improvement for stress tolerance. New Delhi, India: CCSHAU, Hissar & MMB, pp 218–232
Jaligot E, Rival A, Beule T, Dussert S, Verdeil JL (2000) Somaclonal variation in oil palm (Elaeis guineensis Jacq.): the DNA methylation hypothesis. Plant Cell Rep 19:684–690
Joyce SM, Cassells AC (2002) Variation in potato microplant morphology in vitro and DNA methylation. Plant Cell Tissue Organ Cult 70:125–137
Kaeppler SM, Phillips RL (1993a) DNA methylation and tissue culture-induced variation in plants. In Vitro Cell Dev Biol Plant 29:125–130
Kaeppler SM, Phillips RL (1993b) Tissue culture-induced DNA methylation variation in maize. Agric Sci 90:8773–8776
Kaeppler SM, Kaeppler HF, Rhee Y (2000) Epigenetic aspects of somaclonal variation in plants. Plant Mol Biol 43:179–188
Kazazian HH (2004) Mobile elements: drivers of genome evolution. Science 303:1626–1632
Keyte AL, Percifield R, Liu B, Wendel JF (2006) Intraspecific DNA methylation polymorphism in cotton (Gossypium hirsutum). J Hered 97:444–450
Kidwell AG, Lisch D (1997) Transposable elements as source of variation in animals and plants. Proc Natl Acad Sci USA 94:7704–7711
Krishnaraj S, Vasil IK (1995) Somatic embryogenesis in herbaceous monocots. In: Thorpe TA (ed) In vitro embryogenesis in plants. Kluwer, Dordrecht, The Netherlands, pp 417–471
Kumar PS, Mathur VL (2004) Chromosomal instability in callus culture of Pisum sativum. Plant Cell Tissue Organ Cult 78:267–271
Kuznetsova OI, Ash OA, Hartina GA, Gostimskij SA (2005) RAPD and ISSR analyses of regenerated pea Pisum sativum L. plants. Russ J Genet 41:60–65
Larkin PJ, Scowcroft WR (1981) Somaclonal variation—a novel source of variability from cell cultures for plant improvement. Theor Appl Genet 60:443–455
Li Y, Guo W, Liu X, Shan X, Li F, Zhang Z, Liu B (2006) Efficient micropropagation of Japanese Photinia [Photinia glabra (Thunb.) Maxim.] retaining genetic and epigenetic stability. Propag Ornam Plants 6:149–155
Li X, Yu X, Wang N, Feng Q, Dong Z, Liu L, Shen J, Liu B (2007) Genetic and epigenetic instabilities induced by tissue culture in wild barley (Hordeum brevisubulatum (Trin.) Link). Plant Cell Tissue Organ Cult 90:153–168
Liu ZL, Han FP, Tan M, Shan XH, Dong YZ, Wang XZ, Fedak G, Hao S, Liu B (2004) Activation of a rice endogenous retrotransposon Tos17 in tissue culture is accompanied by cytosine demethylation and causes heritable alteration in methylation pattern of flanking genomic regions. Theor Appl Genet 109:200–209
LoSchiavo F, Pitto L, Giuliano G, Torti G, Nuti-Ronchi V, Marazziti D, Vergara R, Orselli S, Terzi M (1989) DNA methylation of embryogenic carrot cell cultures and its variations as caused by mutation, differentiation, hormones and hypomethylating drugs. Theor Appl Genet 77:325–331
Matthes M, Singh R, Cheah SC, Karp A (2001) Variation in oil palm (Elais guineensis Jacq.) tissue culture-derived regenerants revealed by AFLPs with methylation sensitive enzymes. Theor Appl Genet 102:971–979
May RA, Trigiano RN (1991) Somatic embryogenesis and plant regeneration from leaves of Dendrathema grandiflora. J Am Soc Hortic Sci 116:366–371
McClelland M, Nelson M, Raschke E (1994) Effect of site-specific modification on restriction endonucleases and DNA modification methyltransferases. Nucleic Acids Res 22:3640–3659
Meins F Jr (1983) Heritable variation in plant cell culture. Annu Rev Plant Physiol 34:327–346
Muller E, Brown PTH, Hartke S, Lorz H (1990) DNA variation in tissue-culture-derived rice plants. Theor Appl Genet 80:673–679
Osternack N, Saare-Surminski K, Preil W, Lieberei R (1999) Induction of somatic embryos, adventitious shoots and roots in hypocotyls tissue of Euphorbia pulcherrima Willd. Ex Klotzsch: comparative studies on embryogenic and organogenic competence. J Appl Bot 73:197–201
Parra R, Pastor MT, Pérez-Payá E, Amo-Marco JB (2001) Effect of in vitro shoot multiplication and somatic embryogenesis on 5-methylcytosine content in DNA of Myrtus communis L. Plant Growth Regul 33:131–136
Peraza-Echeverria S, Herrera-Valencia VA, James-Kay A (2001) Detection of DNA methylation changes in micropropagated banana plants using methylation-sensitive amplification polymorphism (MSAP). Plant Sci 161:359–367
Peredo EL, Ángeles Revilla M, Arroyo-García R (2006) Assessment of genetic and epigenetic variation in hop plants regenerated from sequential subcultures of organogenic call. J Plant Physiol 163:1071–1079
Phillips MH, Keppler SM, Olhoft P (1994) Genetic variability of plant tissue cultures: breakdown of normal control. Proc Natl Acad Sci USA 91:5222–5226
Portis E, Acquadro A, Comino C, Lanteri S (2004) Analysis of DNA methylation during germination of pepper (Capsicum annuum L.) seeds using methylation-sensitive amplification polymorphism (MSAP). Plant Sci 166:169–178
Quemada H, Roth EK, Lark KG (1987) Changes in methylation status of tissue cultured soybean cells detected by digestion with the restriction enzymes Hpa II and Msp I. Plant Cell Rep 6:63–66
Reyna-Lopez GE, Simpson J, Ruiz-Herrera J (1997) Differences in DNA methylation patterns are detectable during the dimorphic transition of fungi by amplification of restriction polymorphisms. Mol Gen Genet 253:703–710
Russo VEA, Martienssen RA, Riggs AD (1996) Epigenetic mechanisms of gene regulation. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Skoog F, Miller CO (1957) Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol 54:118–130
Smulders MJM, Rus-Kortekaas W, Vosman B (1995) Tissue culture-induced DNA methylation polymorphisms in repetitive DNA of tomato calli and regenerated plants. Theor Appl Genet 91:1257–1264
Stover RH (1987) Somaclonal variation in Grand Naine and Saba bananas in the nursery and in the field. In: Persley GJ, De Langhe EA (eds), Bananas and plantain breeding strategies. Proceedings of an international workshop, No. 21, Cairns, Australia, 13–17 October 1986. Australian Centre for International Agricultural Research (ACIAR), Canberra, pp 136–139
Sunderland N (1977) Nuclear cytology. In: Street HE (ed) Plant tissue and cell culture. Blackwell, Oxford, pp 177–205
Tanurdzic M, Vaughn MW, Jiang H, Lee TJ, Slotkin RK, Sosinski B, Thompson WF, Doerge RW, Martienssen RA (2008) Epigenomic consequences of immortalized plant cell suspension culture. PLoS Biol 6(12):2880–2895
Tariq M, Paszkowski J (2004) DNA and histone methylation in plants. Trends Genet 20(6):244–251
Thomas E, Bright SWJ, Franklin J, Lancaster V, Miflin BJ (1982) Variation amongst protoplast-derived potato plants (Solanum tuberosum cv, “Maris Bar”). Theor Appl Genet 62:65–68
Thorpe TA, Stasolla C (2001) Somatic embryogenesis. In: Bhojwani SS, Soh WH (eds) Current trends in the embryology of angiosperms. Kluwer, Dordrecht, The Netherlands, pp 279–336
Veilleux RE, Johnson ATT (1998) Somaclonal variation: molecular analysis, transformation, interaction, and utilization. Plant Breed Rev 16:229–268
Vos P, Hogers R, Bleeker M, Reijans M, Vandelee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP—a new technique for DNA-fingerprinting. Nucleic Acids Res 23:4407–4414
Wang L, Huang B, He M, Hao S (1990) Somatic embryogenesis and its hormonal regulation in tissue cultures of Freesia refracta. Ann Bot Lond 65:271–276
Wang YM, Dong ZY, Zhang ZJ, Lin XY, Shen Y, Zhou D, Liu B (2005) Extensive de novo genomic variation in rice induced by introgression from wild rice (Zizania latifolia Griseb.). Genetics 170:1945–1956
Xiong LZ, Xu CG, Maroff MAS, Zhang QF (1999) Patterns of cytosine methylation in an elite rice hybrid and its parental lines, detected by a methylation-sensitive amplification polymorphism technique. Mol Gen Genet 261:439–446
Xu ML, Li XQ, Korban SS (2004) DNA-methylation alterations and exchanges during in vitro cellular differentiation in rose (Rosa hybrida L.). Theor Appl Genet 109:899–910
Young WP, Schupp JM, Keim P (1999) DNA methylation and AFLP marker distribution in the soybean genome. Theor Appl Genet 99:785–792
Acknowledgments
This study was supported by the Program for Changjiang Scholars and Innovative Research Team (PCSIRT) in University (#IRT0519), the National Natural Science Foundation of China (30970280), and a grant of the Jilin provincial government of China (20085030).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, X., Yang, D., Cao, D. et al. In Vitro Micropropagation of Freesia hybrida and the Assessment of Genetic and Epigenetic Stability in Regenerated Plantlets. J Plant Growth Regul 29, 257–267 (2010). https://doi.org/10.1007/s00344-009-9133-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-009-9133-4