Abstract
Optical methods based on quantum cascade lasers (QCLs) are becoming popular in many life science applications. We report on two trace gas detection schemes based on continuous wave QCLs for on-line detection of nitric oxide (NO) at the sub-part-per-billion level by volume (ppbv, 1:10−9), using wavelength modulation spectroscopy (WMS) and Faraday rotation spectroscopy (FRS) at 1894 cm−1 and 1875.73 cm−1, respectively. Several technical incremental steps are discussed to further improve the sensitivity of these methods. Examples are included to demonstrate the merits of WMS-based sensor: direct monitoring of NO concentrations in exhaled breath, and from plants under pathogen attack. A simple hand-held breath sampling device that allows single breath collection at various exhalation flows (15, 50, 100 and 300 mL/s, respectively) is developed for off-line measurements and validated in combination with the WMS-based sensor. Additionally, the capability of plants to remove environmental NO is presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In 1890 Alfred Nobel’s physicians prescribed nitroglycerine (a key component of dynamite) as a remedy for his heart disease. For more than 100 years this was used in the treatment of angina pectoris, although nobody knew the physiological mechanism of action. The pioneering work of Robert Furchgott, Louis Ignarro and Ferid Murad, awarded the Nobel Prize in Physiology or Medicine in 1998, has shown that nitroglycerine acts by releasing nitric oxide (NO) as the therapeutic agent and demonstrated that NO is involved in blood pressure control by triggering the blood vessels to relax and widen.
Starting in 1992 when NO was named the ‘molecule of the year’, the scientific community has realized the biological significance of NO, not only its role as atmospheric pollutant. After the first report of exhaled NO in animals and humans [1], the interest in NO monitoring for clinical applications has tremendously increased, with over 1200 articles in the field of asthma and other pulmonary diseases. This fast progress of NO as a potential non-invasive test for disease evaluation and treatment monitoring was possible because the technology for NO detection was already available on the market; the chemiluminescence analysers were used for measuring NO in air pollution and were easily adopted for exhaled NO analysis. Currently, chemiluminescence devices are considered as the ‘gold standard’, providing sufficient accuracy and precision. However, they are bulky and expensive, have high running costs and require technical expertise in calibration, therefore limiting their use in routine patient care. A convenient alternative is offered by the electrochemical sensors, but despite their lower cost and possibility for development of portable or hand-held devices, they suffer from a lack of sensitivity (detection limit > 5 ppbv) and efficiency in NO multiple flow analysis [2–4].
The quantum cascade laser (QCL) technology has rapidly advanced during the last years, offering narrow linewidth, high power at room temperature and continuous wave (cw) operation at mid-IR wavelengths (3–24 μm) [5, 6]. These features are nowadays combined with a wide collection of non-invasive optical methods for selective, sensitive and accurate analysis of NO concentrations [6–9].
Novel mid-IR gas sensors, especially using QCLs as the source, are gaining popularity within the medical/clinical community. Several medical reports have shown their high performances in terms of sensitivity, selectivity and accuracy in comparison to other technologies [10]. Another great advantage offered by optical sensors is the possibility to perform multiple flow rate analysis (exhaled NO concentration is flow dependent) without modification of the sensor [4].
Although most reported research on NO detection is performed in animals and humans, nitric oxide is also a signalling molecule in plants. The last decade has brought much new insight into the pathways of NO production and function in plants. Nevertheless, there are still many details of the NO story to be elucidated. This lack of knowledge is partially due to the technical difficulties in measuring and quantifying NO. Conventional chemiluminescence detection requires higher flow rates, 12 L/h or greater, to achieve a typical detection limit of 1 ppbv. This implies that the NO production from the plant material should be least 12 ppbv, otherwise the NO concentration is barely visible due to the dilution effect. With the optical sensors this limitation is overcome; lower flows of a few litres per hour are easily possible. An updated review describing the advantages and disadvantages of several available methods to detect NO in plants, including laser-based spectroscopy, has been recently published [11].
In this paper, two techniques are presented in detail, using QCL in combination with wavelength modulation spectroscopy (WMS) and Faraday rotation spectroscopy (FRS), for NO detection in both humans and plants. The WMS-based sensor was applied for on-line monitoring of NO concentration during single exhalation. Possibilities for off-line measurements are also considered; a simple hand-held device for sampling breath in a standard way is developed and validated. Moreover, the suitability of WMS-based sensors for accurate detection of NO in planta is shown in two important plant research areas, pathogen infection and NO removal from the atmosphere.
2 Quantum cascade laser-based spectroscopy for NO detection
Continuous wave QCLs (cw-QCLs) are becoming increasingly popular sources for IR chemical sensing. Nowadays, operating at ambient temperatures with high output powers and excellent spectral quality, thermoelectrically cooled cw-QCLs have created a range of novel mid-IR gas sensors offering high sensitivity, selectivity and fast-response concentration measurements. Moreover, new applications for gas sensing are emerging due to their compact size, robust construction and low power requirements.
QCLs emitting in the NO fundamental vibration-rotation band (centred at 5.2 μm) have in recent years triggered the development of QCL-based NO sensors using various spectroscopic methods including direct absorption spectroscopy using multi-pass cells [12], wavelength modulation spectroscopy [13], photoacoustic spectroscopy [14], FRS [15] and high-finesse cavities for extreme absorption path length, i.e. cavity ring-down spectroscopy and on/off-axis integrated cavity output spectroscopy [16, 17].
2.1 Wavelength modulation spectroscopy
In this work, a cw-QCL (Alpes Lasers, Switzerland) operating at −25 °C in the wavelength region of 5.2 μm was combined with an astigmatic multi-pass cell with an effective optical path length of 76 m (AMAC-76, Aerodyne Research) and a wavelength modulation technique for the detection of NO below the part-per-billion by volume (ppbv) level. The details of the sensor configuration and performance have been described elsewhere [7]. The pressure inside the multi-pass cell was about 70 mbar. To study fast events, like on-line breath monitoring, the refresh time of the cell could be decreased to 2 s with a flow rate of 40 L/h, limited by the vacuum pump. Low flows through the cell (<5 L/h) were also possible to avoid dilution of the studied sample when low NO concentrations are expected, as found when determining the NO production of plants. By modulating the injection current of the laser at a frequency of 100 kHz, the absorption signal was transposed at a higher frequency where noise sources are weaker. The absorption spectra were retrieved by demodulation of the signal at the second harmonic (2f) using a lock-in amplifier (model SR844, Stanford Research Systems) with a time constant of 100 μs. The room temperature detector (PV-6, Vigo System, \(D^{*} > 4\times 10^{8}~\mathrm{cm\,Hz}^{1/2}/\mathrm{W}\)) previously used [7] was replaced by a thermoelectrically cooled detector (PVI-4TE, Vigo System), with a three-order-of-magnitude greater sensitivity (\(D^{*} > 10^{11}~\mathrm{cm\,Hz}^{1/2}/\mathrm{W}\)). The use of a detector of higher sensitivity was essential in reducing the optical fringes. While many elements in the optical sensor can cause such fringing, the multi-pass absorption cell was the strongest limitation of the long-term sensitivity. Good beam quality and proper alignment of the laser beam entering the multi-pass cell were obvious requirements, while the highest sensitivity of detection allowed us to decrease the laser beam waist in the cell, thereby improving the long-term stability of the sensor.
The Allan variance plot showing the noise level of the detected background as a function of the integration time of the sampling is displayed on Fig. 1. For both detectors, a detection limit of 0.5 ppbv with 1 s averaging was achieved. The best noise level of 0.05 pptv was obtained for 10 min averaging time with the cooled detector.
WMS on a QCL emitting in the strongest absorption region of NO and coupled with a multi-pass cell has led to the development of a simple, robust and sensitive sensor suitable for detection of NO in multiple applications. The present sensitivity is appropriate for both applications described in this work, and it is comparable with other ongoing optical sensor developments [18, 19], even though it does not represent the ultimate sensitivity (comparison in terms of absorption path length). For breath analysis, the detection limit of 0.5 ppbv in 1 s is sufficient for on-line monitoring of the low ppbv NO concentrations in the exhaled breath, whilst a better sensitivity can be achieved for plant studies by averaging the signal.
2.2 Faraday rotation spectroscopy
Most of the QCL-based NO sensors employ sources emitting in the 5.2 μm region due to the presence of the strongest NO absorption band. However, the presence of water (H2O) and carbon dioxide (CO2) in this region can disturb the spectroscopic measurements of NO concentrations. Therefore, it is essential to eliminate the influence of these interferences, especially when plant or breath samples are analysed. From this point of view, Faraday rotational spectroscopy (FRS) seems to be the most favourable among the spectroscopic techniques, because it is not sensitive to diamagnetic molecules such as H2O or CO2. The technique, first described in the 1980s [20], appears to be a powerful and versatile method for quantitative and selective detection of paramagnetic molecules, such NO, O2 or OH−. For NO sensing with FRS, the 5.3 μm region is commonly used [15, 21, 22].
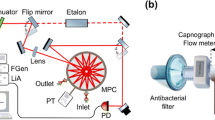
The experimental set-up in FRS employing a multi-pass cell is shown in Fig. 2. The QCL (Alpes Lasers, Switzerland) was thermoelectrically cooled up to −25 °C and generated about 1 mW output power. The current of the QCL was modulated by a 20 Hz triangular signal to scan the Q(5/2) NO transition at 5.33 μm (1875.73 cm−1). The light from the QCL was sent to the astigmatic multi-pass cell (AMAC-76, Aerodyne, USA) with a path length of 76 m, 0.5 litre volume and 80 mbar internal pressure. The cell was placed inside a 25 cm long solenoid producing a modulated magnetic field of 100 G at a frequency of 100 Hz. Since the technical noise is frequency dependent, to improve the field strength at high frequency modulation, a RLC circuit with a resonant frequency of 6 kHz was implemented.
Schematic representation of the multi-pass-cell-based FRS spectrometer for NO detection at 1875.73 cm−1. The QCL beam was sent to the astigmatic multi-pass cell placed inside a 25 cm long solenoid producing a modulated magnetic field of 100 G at 100 Hz. Two Rochon polarisers (MgF2 prisms, extinction ratio 10−5) are placed before the entrance of the cell (polariser) and after it (analyser). The beam is focused on a photovoltaic room temperature IR detector, and the output signal is demodulated by a lock-in amplifier and analysed by the LabView program
The QCL beam passed through two Rochon polarisers (MgF2 prisms, extinction ratio 10−5). The first polariser was placed before entering the multi-pass cell and filters out unwanted directions of light polarisation, while the second polariser (analyser) was placed after the cell almost perpendicular to the first one. The angle between the two polarisers was adjusted in order to optimize the signal-to-noise ratio (SNR) of the signal—a trade-off between laser noise and electronic noise of the detection system. The beam was focused on a photovoltaic room temperature IR detector (PV-5, \(D^{*}= 9\times 10^{8}~\mathrm{cm\,Hz}^{1/2}/\mathrm{W}\), τ=20 ns, Vigo System). The output signal was demodulated by a lock-in amplifier (EG&G Princeton Applied Research 5209, USA), acquired via an acquisition card (National Instruments BNC-210, USA) and analysed by the LabView program.
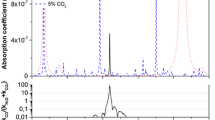
Figure 3 shows a HITRAN simulated FRS signal for 1 ppm concentration of NO in N2 for a 76 m path length at 80 mbar pressure and the measured FRS signal under the same conditions at 1875.73 cm−1 (average of 20 scans during 1 s). The wavelength scanning range was limited by the technical specifications of the laser. In Fig. 4 the Allan variance plot showing the noise level of the detected signal as a function of the integration time of the FRS sampling is displayed. The detection limit was 9 ppbv of NO in N2 for 1 s averaging, and the minimum detection limit corresponded to 2.3 ppbv for an averaging time of 16 s.
Recently, the development of high performance FRS spectrometers has been reported [15, 23]. The ultimate sensitivity levels achieved in these experiments was 4.7 and 4.5 ppbv for 1 s averaging time, respectively. In comparison, there are several factors affecting the sensitivity of our present set-up. First, the NO absorption strength at the wavelength of the available QCL is twice as low as the most sensitive transition for FRS (1875.81 cm−1) [15, 21, 22]. The power of the QCL is only 1 mW compared to those reported in the literature: 3 mW and 60 mW, respectively [15, 23]. In addition, the detectivity (D∗) of the detectors used in these studies is 2 orders of magnitude higher than in the present experiment. Therefore, by using a more suitable (wavelength) QCL and detector for FRS, the predicted minimum detection limit can be improved by a factor 10, corresponding to a sub-ppbv detection level for 1 s acquisition time.
3 NO detection from human breath
The first application presented here refers to NO monitoring from human breath. The measurement of NO is becoming more widely accepted for diagnosis or monitoring, recognized as a cost-effective means of non-invasively assessing asthma, airway inflammation, etc.
Higher levels of NO have been demonstrated in asthmatic patients [24, 25], and corticosteroid therapy has been shown to decrease NO in breath [26]. Nitric oxide is mainly synthesized in the epithelial layer in the conducting airways, and since the production of NO is different along them, the concentration in exhaled air will highly depend on the exhalation flow rate. The highest production of NO is from nasal tissues, and the lowest contribution is from the alveoli. Since exhaled NO is flow-dependent, its measurement at a single flow rate (50 mL/s) is nowadays a widely used standard in clinical applications [27]. However, applying the two-compartment model to multiple flow rates measurements [28] may provide (additional) diagnostic information in respiratory diseases where single-breath measurements are ambiguous, by segregating exhaled NO coming from alveolar and airway regions [29, 30]. Three main NO parameters can be determined: the airway wall concentration of NO (CawNO in ppbv), the diffusing capacity of NO (DawNO in ppbv/s) and a steady-state alveolar NO concentration (CANO in ppbv). To obtain these terms during large clinical studies, an efficient and simple breath sampler was developed and coupled with a sensitive WMS-based sensor.
Currently there are two commercial laser-based instruments for the determination of exhaled NO, using a lead salt laser [31] and a QCL [10], respectively. The WMS-based sensor described in Sect. 2.1 has been successfully compared with other detection methods for NO measurements [4]. By measuring the same samples from asthmatic children, the NO values were found to be in good agreement (within 1.1 ppbv) between the WMS-based and chemiluminescent-based sensor, and a 1.7 ppbv difference was observed when compared to the electrochemical sensor. The sensor has also been demonstrated to be efficient for exhaled NO measurements collected at various flow rates by performing either on-line or off-line sampling. This work indicated that the WMS-based sensor can be applied to determine flow-independent parameters in human clinical trials.
As proof of concept, the WMS-based sensor was coupled to a commercially available breath sampler (Loccioni, Italy). The sampler helped the patient to maintain constant mouth pressure and therefore constant exhalation flow according to the ATS/ERS recommendations [32]. At the same time, the CO2 concentration level was recorded, as this can provide useful information either to check the quality of the exhalation or to normalize the NO signal. The NO measurements at different flows can be assessed by changing the resistance of the sampler. Figure 5 shows a typical record performed with the Loccioni sampler connected to the laser-based sensor at an exhalation flow of 50 mL/s. The NO signal was displayed every 1 s. The response time of the WMS-based sensor was a few seconds, because of the high flow rates going through the multi-pass cell. Therefore, on-line single-breath monitoring of NO could be performed, and real-time NO concentration was provided.
On-line capabilities of the WMS-based sensor coupled with the commercial Loccioni sampler. Three parameters are recorded at the same time during on-line exhalation, namely the mouth pressure to ensure constant flow (on top), the CO2 concentration (blue curve) and the exhaled NO concentration (red curve)
The advantages of on-line sampling are obvious; alternatively, off-line breath sampling is to be considered when analysing large numbers of samples, which is the case for clinical studies. For off-line measurement, a new custom built hand-held breath collection device has been developed (Fig. 6). The breath collection device was designed according to the guidelines of the ATS/ERS for the standardized collection of exhaled NO [32]. The sampler is simple, extremely user-friendly, and can be used by nurses and unspecialized medical personnel. It consists of a mouthpiece, a pressure meter, a Teflon piece for flow resistance and a sampling bag. Additionally, a discard bag can be considered to remove the first part of the breath. A constant exhalation flow rate was maintained by monitoring the mouth pressure. For the breath collection, patients were asked to take a deep breath and perform one single exhalation into the mouthpiece. Three LEDs were giving feedback to the patient to maintain a constant mouth pressure, which was set to 10 mbar, enough to close the soft palate and to avoid the need for a nose clip.The pressure range indicated by the LEDs was set by the pressure meter to ±5 %. Breaths were collected at various constant exhalation flows from each subject by easily changing the resistance piece. All exhalation flows (15, 50, 100 and 250 mL/s) were performed at the same mouth pressure of 10 mbar. The calibration of the sampler was made with a mass flow meter (Brook Instrument) ranging from 0 to 30 slpm (standard litre per minute).
Custom made hand-held breath sampler. (a) Mouth piece. (b) Pressure meter with 3 LEDs helping the person to maintain constant exhalation flow. (c) Fluoroplastic tube (PFA). (d) Teflon piece. By changing the diameter of the hole, different flow rates can be assessed, keeping the mouth pressure of 10 mbar. (e) Mylar balloon
In order to validate the sampler, comparisons were made with the Loccioni sampler at four different flows. Ten healthy subjects participated in this validation study. At each flow, every subject was asked to exhale with both samplers. The breath samples were collected in NO-impermeable aluminium-foil bags of 500 ml capacity (Mylar balloon, ABC Ballonnen, Zeist, The Netherlands). Two replicate experiments were conducted.
Figure 7 shows the measured NO concentrations obtained using the custom made hand-held breath sampler versus the NO concentrations using the commercial Loccioni sampler. In total, 16 samples per person are considered (4 flows × 2 samplers × 2 repetitions). A slope of 0.986 with R 2=0.993 demonstrates a good correlation between the two devices.
Comparison between the off-line measured NO concentrations with the WMS-based sensor using the Loccioni sampler and the hand-held sampler depicted in Fig. 6. Each patient was asked to give a single exhalation breath with both samplers. The experiment was performed at four different exhalation flows on 10 healthy persons and repeated two times. (R 2=0.993 and slope = 0.986)
4 NO emission from plants
It is now generally accepted that NO plays an important role in plant growth and development, as well as stomatal regulation and protection against biotic or abiotic stresses. However, relatively little is known about its biological role in plants, and the most suitable method to measure NO production is still disputed. An updated review describing the advantages and disadvantages of several available methods to detect NO, including laser-based spectroscopy, has been recently published [11].
4.1 Detection of NO from pathogen-infected plants
Plant disease remains a major source of crop loss throughout the world and thus hinders efforts to achieve food security [33, 34]. The increases in crop production in the West have come in part through the use of agricultural biocides. Although biocide use will undoubtedly continue to be useful, deleterious effects on human health and the environment necessitate the deployment of alternative strategies of crop protection [35]. The breeding of disease-resistant varieties of crop plants is also an important strategy; various resistance (R) genes able to recognise the pathogen are bred into agriculturally important varieties, a process which can take many years. It is also important to understand how host defences neutralise infections, as there are many diseases where effective R genes have yet to be discovered. The most visible type of plant defence is the elicitation of a highly localised programmed cell death known as the hypersensitive response (HR). Classically, the HR was thought to act to confine the pathogen to the site of infection, but now it is considered to be more a consequence than a cause of defence [36].
Nitric oxide has emerged as an important component of HR-associated defences, so if its production is reduced, the plant resistance may be compromised [37, 38]. This stated, actual measurements of NO production from pathogen-challenged plants have often employed NO-sensitive fluorescent dyes whose specificity has occasionally been contested [11]. However, in 2005, we provided the first on-line and in planta demonstration of NO production from pathogen-infected tobacco leaves using CO laser-based photoacoustic detection [38].
This preliminary work was followed by a similar study exploiting the model plant species Arabidopsis thaliana, which offers a range of genetic resources (mutants and transgenic lines) through which NO-mediated effects can be dissected. Infiltration of cells of the bacterial pathogen Pseudomonas syringae pathovar tomato (Pst) genotype AvrRpm1 suspended in 10 mM MgCl2 using a syringe elicited an HR-type cell death at the point of inoculation in isolated leaves of A. thaliana (Fig. 8a). Conversely, infiltration with 10 mM MgCl2 alone resulted in no visible response by the plant. As was previously observed in tobacco following challenge with P.s.pv. phaseolicola [38], a rapid and sustained increase in NO production in the pathogen inoculated leaves only was detected using the WMS-based system described in Sect. 2.1 (Fig. 8b). This further demonstrates the complexity and subtlety of plant pathogen interactions and the possibility that elevated NO production could be used to enhance disease resistance. It also shows the value of accurate in planta NO detection using WMS-based systems.
Nitric oxide production from pathogen-challenged plants as detected using WMS-based system. (a) 24 h following infection: isolated leaves from Arabidopsis thaliana Col-0 were infiltrated with Pseudomonas syringae pv. tomato DC3000 (Pst) AvrRpm1 (2×106 bacterial cells per mL in 10 mM MgCl2 using a needleless syringe (top). Uninfected leaves exhibit barely noticeable symptoms (bottom). (b) NO detection from five Arabidopsis leaves infected with bacteria (see a) compared to five uninfected leaves (control)
4.2 Do plants filter environmental NO?
In the experiment described above (Fig. 8), isolated pathogen-challenged leaves were placed in the cuvette so that NO production could be measured. However, during attempts to monitor NO production of intact plants (i.e. the whole plant including its pot and soil) in response to pathogen inoculation, a high NO production was detected even for uninoculated plants (Fig. 9a). Further investigation revealed the soil and roots to be a significant source of NO. When the plant was excised at the roots and release of NO from the soil was assessed, it was observed that NO production was further increased by removal of the aerial plant tissues. When excised plant tissues were returned to the cuvette, NO production was again reduced, suggesting that leaf tissues have the capacity to remove NO from the atmosphere.
Removal of atmospheric NO by plants. (a) NO production from a 5 week old Arabidopsis thaliana Col-0 plant in pot. Cycles 1–3 = entire system plant (plant + soil pot), Cycles 4, 12 = empty cuvette, Cycles 5–7 = soil pot following careful cutting of the plant at stem-root junction, Cycles 8–11 = soil following reapplication of cut plant onto the top of the soil, Cycles 13–15 = soil pot following removal of cut plant. (b) Measurements of NO productions from single core samples of 10×10 cm wide ×20 cm deep farm grassland soil; whole cores (white bars) and cores where the plant later has been removed (black bars, referred to as ‘decapitated’)
NO production mediated by soil microbes is a known feature of the oxido-reductive steps from NH4 to NO3 (nitrification and denitrification) that comprise the nitrogen cycle [39]. Past investigations of the removal of NO and other airborne pollutants have suggested that plants may have some capacity to absorb atmospheric NO [40, 41], but we are not aware of any investigations of the ability of plants to remove NO formed naturally by soils. Thus, we hypothesized that plant material could remove NO produced from soil. To test this, a concentration of 500 ppbv of NO in air was flushed a constant flow through a glass cuvette. When an excised A. Thaliana rosette (without roots) was placed in a cuvette, the concentration of NO detected using the WMS system described above was reduced by 50 %. When the rosette was removed from the cuvette and briefly microwaved (5 s in a commercial microwave oven) and reanalysed in the cuvette, the NO absorbing ability of the leaves was lost. This indicated that the ability of plants to remove atmosphere NO was heat-labile and possibly enzymatically mediated, since leaf enzymes would be inactivated in the microwave step. This serendipitous observation could indicate an important environmental phenomenon.
In nature, NO is readily oxidized to form NO2; with NO and NO2 together usually referred to as NO x pollutants. The NO x gases are best known as a by-product of vehicle pollution, but should also be considered as a feature of the rural environment, particularly following the addition of nitrate fertilizers to soils. The presence of NO x is essential for O3 formation, so NO removal by plants could play a key role in reducing tropospheric O3.
The possibility that vegetation can mitigate NO release from agricultural soils was further explored using soil mesocosms (10×10 cm wide × 20 cm deep monoliths) from a sheep-grazed grassland at Bronydd Mawr Research Farm (51°37′N 03°38′W) near Brecon in Powys, Wales UK. The soils overlie the Old Red Sandstone and are predominantly well-drained acid brown earths.
Cores from fertiliser-treated (160 kg N/ha/yr, similar to the levels applied agriculturally for upland sheep pasture) and control plots (no inputs since 1991) both grazed to 4 cm sward height were studied. Measurement of NO production was made from the cores (with vegetation intact) following transport to Nijmegen. Release of NO was detected from all cores but showed considerable variation, most likely due to variations in soil moisture content which in turn was influenced by hydrological differences between plots (Fig. 9b).
Crucial to our hypothesis, when plant material was cut from the cores at ground level and removed from the cuvettes (referred to as ‘decapitated’ in Fig. 9b), in the majority of cases more NO production was measured. Although, decapitation could have influenced NO production by soil microbes due to altered oxygen status, it also suggests that vegetation may play an important role in the absorption of soil-generated NO. In a forthcoming paper, we will describe how NO removal by plants is dependent on endogenous haemoglobins.
5 Conclusions and future outlook
Important achievements have been made in medicine and plant sciences through NO analysis. This should be continued, since new avenues are now open and interesting unexplored directions are offered to life science researchers. Exhaled NO in humans is a promising diagnostic and treatment monitoring tool that, in combination with a standard method for breath collection, has the potential to gain clinical acceptance. NO modelling over single flow rate is emerging as it provides a route towards non-invasive early detection of lung diseases and targets more specific treatments. The role of NO in plants as a signalling molecule related to various processes as well as the capability of plants to remove environmental NO represent new fields to be investigated.
The development of reliable, user friendly and less expensive NO sensors is thus an important step to ensure progress in all these fields. The potential applications of NO in the future will rely on well-validated and accurate instruments, and so far, optical techniques using QCLs have been shown to fulfil the demands for monitoring NO emission from human breath and plants. We presented here the development of two techniques, WMS and FRS, as suitable candidates for such applications. The advantages over the conventional method (chemiluminescence) such as direct NO detection, sensitivity and selectivity were key elements. Measurements of exhaled NO at multiple flows can be done easily with one sensor in both on-line and off-line configurations. The development of a compact and user-friendly breath sampler for multiple flow analysis may trigger studies in the intensive care unit, bringing new opportunities for NO analysis in disease diagnosis and treatment. Monitoring NO production from plant material and small changes in the dynamics of NO plant emission is now possible.
References
L.E. Gustafsson, A.M. Leone, M.G. Persson, N.P. Wiklund, S. Moncada, Biochem. Biophys. Res. Commun. 181, 852 (1991)
Z. Borrill, D. Clough, N. Truman, J. Morris, S. Langley, D. Singh, Respir. Med. 100, 1392 (2006)
M.R. McCurdy, Y. Bakhirkin, G. Wysocki, F.K. Tittel, J. Biomed. Opt. 12, 034034 (2007)
J. Mandon, M. Hogman, P.J.F.M. Merkus, J.G.C. Van Amsterdam, F.J.M. Harren, S.M. Cristescu, J. Biomed. Opt. 17, 017003 (2012)
F.K. Tittel, D. Richter, A. Fried, in Solid-State Mid-Infrared Laser Sources, ed. by I.T. Sorokina, K.T. Vodopyanov (Springer, Berlin, Heidelberg, 2003)
R.F. Curl, F. Capasso, C. Gmachl, A.A. Kosterev, B. McManus, R. Lewicki, M. Pusharsky, G. Wysocki, F.K. Tittel, Chem. Phys. Lett. 487, 1 (2010)
S.M. Cristescu, S.T. Persijn, S.T.L. Hekkert, F.J.M. Harren, Appl. Phys. B 92, 343 (2008)
T.H. Risby, F.K. Tittel, Opt. Eng. 49, 111123 (2010)
L. Menzel, A.A. Kosterev, R.F. Curl, F.K. Tittel, C. Gmachl, F. Capasso, D.L. Sivco, J.N. Baillargeon, A.L. Hutchinson, A.Y. Cho, W. Urban, Appl. Phys. B 72, 859 (2001)
J.H. Shorter, D.D. Nelson, J.B. McManus, M.S. Zahniser, S.R. Sama, D.K. Milton, J. Breath Res. 5 (2011)
L.A.J. Mur, J. Mandon, S.M. Cristescu, F.J.M. Harren, E. Prats, Plant Sci. 181, 509 (2011)
J.B. McManus, J.H. Shorter, D.D. Nelson, M.S. Zahniser, D.E. Glenn, R.M. Mcgovern, Appl. Phys. B 92, 387 (2008)
B.W.M. Moeskops, S.M. Cristescu, F.J.M. Harren, Opt. Lett. 31, 823 (2006)
V. Spagnolo, A.A. Kosterev, L. Dong, R. Lewicki, F.K. Tittel, Appl. Phys. B 100, 125 (2010)
R. Lewicki, J.H. Doty, R.F. Curl, F.K. Tittel, G. Wysocki, Proc. Natl. Acad. Sci. USA 106, 12587 (2009)
A.A. Kosterev, A.L. Malinovsky, F.K. Tittel, C. Gmachl, F. Capasso, D.L. Sivco, J.N. Baillargeon, A.L. Hutchinson, A.Y. Cho, Appl. Opt. 40, 5522 (2001)
D.S. Baer, J.B. Paul, J.B. Gupta, A. O’Keefe, Appl. Phys. B 75, 261 (2002)
S.P. Mondal, P.K. Dutta, G.W. Hunter, B.J. Ward, D. Laskowski, R.A. Dweik, Sens. Actuators B, Chem. 158, 292 (2011)
L. Dong, V. Spagnolo, R. Lewicki, F.K. Tittel, Opt. Express 19, 24037 (2011)
G. Litfin, C.R. Pollock, R.F. Curl, F.K. Tittel, J. Chem. Phys. 72, 6602 (1980)
H. Ganser, W. Urban, A.M. Brown, Mol. Phys. 101, 545 (2003)
T. Fritsch, M. Horstjann, D. Halmer, P. Sabana, P. Hering, M. Murtz, Appl. Phys. B 93, 713 (2008)
P. Kluczynski, S. Lundqvist, J. Westberg, O. Axner, Appl. Phys. B 103, 451 (2011)
P.J. Barnes, Thorax 51, 218 (1996)
K. Alving, E. Weitzberg, J.M. Lundberg, Eur. Respir. J. 6, 1368 (1993)
A. Zacharasiewicz, Paediatr. Respir. Rev. 8, 94 (2007)
R.A. Dweik, P.B. Boggs, S.C. Erzurum, C.G. Irvin, M.W. Leigh, J.O. Lundberg, A.C. Olin, A.L. Plummer, D.R. Taylor, A.T.S.C. Interpretat, Am. J. Respir. Crit. Care Med. 184, 602 (2011)
N.M. Tsoukias, S.C. George, J. Appl. Physiol. 85, 653 (1998)
A.P. Pietropaoli, I.B. Perillo, A. Torres, P.T. Perkins, L.M. Frasier, M.J. Utell, M.W. Frampton, R.W. Hyde, J. Appl. Physiol. 87, 1532 (1999)
M. Hogman, N. Drca, C. Ehrstedt, P. Merilainen, Respir. Med. 94, 985 (2000)
K. Namjou, C.B. Roller, T.E. Reich, J.D. Jeffers, G.L. McMillen, P.J. McCann, M.A. Camp, Appl. Phys. B 85, 427 (2006)
American Thoracic Society and European Respiratory Society, Am. J. Respir. Crit. Care Med. 171, 912 (2005)
B. Baker, P. Zambryski, B. Staskawicz, S.P. DineshKumar, Science 276, 726 (1997)
R.N. Strange, P.R. Scott, Annu. Rev. Phytopathol. 43, 83 (2005)
D. Tilman, K.G. Cassman, P.A. Matson, R. Naylor, S. Polasky, Nature 418, 671 (2002)
L.A.J. Mur, P. Kenton, A.J. Lloyd, H. Ougham, E. Prats, J. Exp. Bot. 59, 501 (2008)
M. Delledonne, Y.J. Xia, R.A. Dixon, C. Lamb, Nature 394, 585 (1998)
L.A.J. Mur, I.E. Santosa, L.J.J. Laarhoven, N.J. Holton, F.J.M. Harren, A.R. Smith, Plant Physiol. 138, 1247 (2005)
E.A. Davidson, L.V. Verchot, Global Biogeochem. Cycles 14, 1035 (2000)
A.C. Hill, J. Air Pollut. Control Assoc. 21, 341 (1971)
A. Neubert, D. Kley, J. Wildt, H.J. Segschneider, H. Forstel, Atmos. Environ. 27A, 2137 (1993)
Acknowledgements
This work was supported by the GO-EFRO Ultragas gas analysis systems for quality control of agricultural products and medical diagnostics, project no 2009-010034 of the province of Gelderland (The Netherlands) and EU, EU-Marie Curie fellowships EU-people-2010-IEF, project no 275584 QCLaser Nose and the EU-FP6-Infrastructures-5 program, project FP6-026183 ‘Life Science Trace Gas Facility’. We thank the anonymous reviewers for their useful comments.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Cristescu, S.M., Marchenko, D., Mandon, J. et al. Spectroscopic monitoring of NO traces in plants and human breath: applications and perspectives. Appl. Phys. B 110, 203–211 (2013). https://doi.org/10.1007/s00340-012-5050-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-012-5050-5