Abstract
Objectives
To explore the feasibility of using amide proton transfer-weighted (APTw) MRI metrics as surrogate biomarkers to identify the O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status in glioblastoma (GBM).
Methods
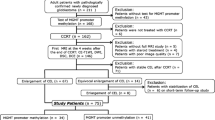
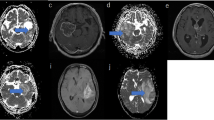
Eighteen newly diagnosed GBM patients, who were previously scanned at 3T and had a confirmed MGMT methylation status, were retrospectively analysed. For each case, a histogram analysis in the tumour mass was performed to evaluate several quantitative APTw MRI metrics. The Mann-Whitney test was used to evaluate the difference in APTw parameters between MGMT methylated and unmethylated GBMs, and the receiver-operator-characteristic analysis was further used to assess diagnostic performance.
Results
Ten GBMs were found to harbour a methylated MGMT promoter, and eight GBMs were unmethylated. The mean, variance, 50th percentile, 90th percentile and Width10-90 APTw values were significantly higher in the MGMT unmethylated GBMs than in the MGMT methylated GBMs, with areas under the receiver-operator-characteristic curves of 0.825, 0.837, 0.850, 0856 and 0.763, respectively, for the discrimination of MGMT promoter methylation status.
Conclusions
APTw signal metrics have the potential to serve as valuable imaging biomarkers for identifying MGMT methylation status in the GBM population.
Key Points
• APTw-MRI is applied to predict MGMT promoter methylation status in GBMs.
• GBMs with unmethylated MGMT promoter present higher APTw-MRI than methylated GBMs.
• Multiple APTw histogram metrics can identify MGMT methylation status.
• Mean APTw values showed the highest diagnostic accuracy (AUC = 0.825).




Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- APTw:
-
Amide proton transfer-weighted
- AUC:
-
Area under the curve
- CEST:
-
Chemical exchange-dependent saturation transfer
- FLAIR:
-
Fluid-attenuated inversion recovery
- GBM:
-
Glioblastoma
- Gd-T1w:
-
Gadolinium-enhanced T1-weighted
- MGMT:
-
O6-methylguanine-DNA methyltransferase
- MRI:
-
Magnetic resonance imaging
- ROC:
-
Receiver operator characteristic curve
- T1w:
-
T1-weighted
- T2w:
-
T2-weighted
References
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Claes A, Idema AJ, Wesseling P (2007) Diffuse glioma growth: a guerilla war. Acta Neuropathol 114:443–458
Zhang J, Stevens MF, Laughton CA, Madhusudan S, Bradshaw TD (2010) Acquired resistance to temozolomide in glioma cell lines: molecular mechanisms and potential translational applications. Oncology 78:103–114
Ramirez YP, Weatherbee JL, Wheelhouse RT, Ross AH (2013) Glioblastoma multiforme therapy and mechanisms of resistance. Pharmaceuticals (Basel) 6:1475–1506
Woods D, Turchi JJ (2013) Chemotherapy induced DNA damage response: convergence of drugs and pathways. Cancer Biol Ther 14:379–389
Choi C, Ganji S, Hulsey K et al (2013) A comparative study of short- and long-TE (1)H MRS at 3 T for in vivo detection of 2-hydroxyglutarate in brain tumors. NMR Biomed 26:1242–1250
Ahmed SU, Carruthers R, Gilmour L, Yildirim S, Watts C, Chalmers AJ (2015) Selective Inhibition of Parallel DNA Damage Response Pathways Optimizes Radiosensitization of Glioblastoma Stem-like Cells. Cancer Res 75:4416–4428
Hegi ME, Diserens AC, Gorlia T et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003
Zhao F, Li M, Kong L, Zhang G, Yu J (2016) Delineation of radiation therapy target volumes for patients with postoperative glioblastoma: a review. Onco Targets Ther 9:3197–3204
Weller M, Felsberg J, Hartmann C et al (2009) Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol 27:5743–5750
Sarkaria JN, Kitange GJ, James CD et al (2008) Mechanisms of chemoresistance to alkylating agents in malignant glioma. Clin Cancer Res 14:2900–2908
Weller M, Stupp R, Reifenberger G et al (2010) MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol 6:39–51
Pope WB, Chen JH, Dong J et al (2008) Relationship between gene expression and enhancement in glioblastoma multiforme: exploratory DNA microarray analysis. Radiology 249:268–277
Ellingson BM (2015) Radiogenomics and imaging phenotypes in glioblastoma: novel observations and correlation with molecular characteristics. Curr Neurol Neurosci Rep 15:506
Drabycz S, Roldan G, de Robles P et al (2010) An analysis of image texture, tumor location, and MGMT promoter methylation in glioblastoma using magnetic resonance imaging. Neuroimage 49:1398–1405
Korfiatis P, Kline TL, Coufalova L et al (2016) MRI texture features as biomarkers to predict MGMT methylation status in glioblastomas. Med Phys 43:2835–2844
Kassner A, Thornhill RE (2010) Texture analysis: a review of neurologic MR imaging applications. AJNR Am J Neuroradiol 31:809–816
Harris RJ, Cloughesy TF, Liau LM et al (2015) pH-weighted molecular imaging of gliomas using amine chemical exchange saturation transfer MRI. Neuro-Oncol 17:1514–1524
Moon WJ, Choi JW, Roh HG, Lim SD, Koh YC (2012) Imaging parameters of high grade gliomas in relation to the MGMT promoter methylation status: the CT, diffusion tensor imaging, and perfusion MR imaging. Neuroradiology 54:555–563
Corrigan F, Mander KA, Leonard AV, Vink R (2016) Neurogenic inflammation after traumatic brain injury and its potentiation of classical inflammation. J Neuroinflammation 13:264
Gupta A, Omuro AM, Shah AD et al (2012) Continuing the search for MR imaging biomarkers for MGMT promoter methylation status: conventional and perfusion MRI revisited. Neuroradiology 54:641–643
Koyama H, Ikenuma H, Toda H et al (2017) Synthesis of PET probe O6-[(3-[11C]methyl)benzyl]guanine by Pd0-mediated rapid C-[11C]methylation toward imaging DNA repair protein O6-methylguanine-DNA methyltransferase in glioblastoma. Bioorg Med Chem Lett 27:1892–1896
Ward KM, Aletras AH, Balaban RS (2000) A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J Magn Reson 143:79–87
Zhou J, van Zijl PC (2006) Chemical exchange saturation transfer imaging and spectroscopy. Progr NMR Spectr 48:109–136
Zhou J, Payen J, Wilson DA, Traystman RJ, van Zijl PCM (2003) Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nature Med 9:1085–1090
Zhou J, Lal B, Wilson DA, Laterra J, van Zijl PCM (2003) Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med 50:1120–1126
Zhou J, Zhu H, Lim M et al (2013) Three-dimensional amide proton transfer MR imaging of gliomas: Initial experience and comparison with gadolinium enhancement. J Magn Reson Imaging 38:1119–1128
Togao O, Yoshiura T, Keupp J et al (2014) Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neuro-Oncology 16:441–448
Jiang S, Yu H, Wang X et al (2016) Molecular MRI differentiation between primary central nervous system lymphomas and high-grade gliomas using endogenous protein-based amide proton transfer MR imaging at 3 Tesla. Eur Radiol 26:64–71
Yu H, Lou H, Zou T et al (2017) Applying protein-based amide proton transfer MR imaging to distinguish solitary brain metastases from glioblastoma. Eur Radiol:DOI. https://doi.org/10.1007/s00330-00017-04867-z
Jia G, Abaza R, Williams JD et al (2011) Amide proton transfer MR imaging of prostate cancer: A preliminary study. J Magn Reson Imaging 33:647–654
Yuan J, Chen S, King AD et al (2014) Amide proton transfer-weighted imaging of the head and neck at 3 T: a feasibility study on healthy human subjects and patients with head and neck cancer. NMR Biomed 27:1239–1247
Wen Z, Hu S, Huang F et al (2010) MR imaging of high-grade brain tumors using endogenous protein and peptide-based contrast. Neuroimage 51:616–622
Choi YS, Ahn SS, Lee SK et al (2017) Amide proton transfer imaging to discriminate between low- and high-grade gliomas: added value to apparent diffusion coefficient and relative cerebral blood volume. Eur Radiol. https://doi.org/10.1007/s00330-00017-04732-00330
Togao O, Hiwatashi A, Yamashita K et al (2017) Grading diffuse gliomas without intense contrast enhancement by amide proton transfer MR imaging: comparisons with diffusion- and perfusion-weighted imaging. Eur Radiol 27:578–588
Jiang S, Eberhart CG, Zhang Y et al (2017) Amide proton transfer-weighted MR image-guided stereotactic biopsy in patients with newly diagnosed gliomas. Eur J Cancer 83:9–18
Ma B, Blakeley JO, Hong X et al (2016) Applying amide proton transfer-weighted MRI to distinguish pseudoprogression from true progression in malignant gliomas. J Magn Reson Imaging 44:456–462
Park KJ, Kim HS, Park JE, Shim WH, Kim SJ, Smith SA (2016) Added value of amide proton transfer imaging to conventional and perfusion MR imaging for evaluating the treatment response of newly diagnosed glioblastoma. Eur Radiol 26:4390–4403
Oue N, Shigeishi H, Kuniyasu H et al (2001) Promoter hypermethylation of MGMT is associated with protein loss in gastric carcinoma. Int J Cancer 93:805–809
Tee YK, Donahue MJ, Harston GW, Payne SJ, Chappell MA (2014) Quantification of amide proton transfer effect pre- and post-gadolinium contrast agent administration. J Magn Reson Imaging 40:832–838
Zhang Y, Heo HY, Lee DH et al (2016) Selecting the reference image for registration of CEST series. J Magn Reson Imaging 43:756–761
Ling W, Regatte RR, Navon G, Jerschow A (2008) Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST). Proc Natl Acad Sci (USA) 105:2266–2270
Zhou J, Hong X, Zhao X, Gao J-H, Yuan J (2013) APT-weighted and NOE-weighted image contrasts in glioma with different RF saturation powers based on magnetization transfer ratio asymmetry analyses. Magn Reson Med 70:320–327
Jones CK, Huang A, Xu J et al (2013) Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7T. Neuroimage 77:114–124
Heo H-Y, Zhang Y, Lee D-H, Hong X, Zhou J (2016) Quantitative assessment of amide proton transfer (APT) and nuclear Overhauser enhancement (NOE) imaging with extrapolated semi-solid magnetization transfer reference (EMR) signals: Application to a rat glioma model at 4.7 T. Magn Reson Med 75:137–138
Paech D, Zaiss M, Meissner JE et al (2014) Nuclear overhauser enhancement mediated chemical exchange saturation transfer imaging at 7 Tesla in glioblastoma patients. PLoS One 9:e104181
Hua J, Jones CK, Blakeley J, Smith SA, van Zijl PCM, Zhou J (2007) Quantitative description of the asymmetry in magnetization transfer effects around the water resonance in the human brain. Magn Reson Med 58:786–793
Zhou J, Blakeley JO, Hua J et al (2008) Practical data acquisition method for human brain tumor amide proton transfer (APT) imaging. Magn Reson Med 60:842–849
Liang HY, Huang YQ, Yang ZX, Ying D, Zeng MS, Rao SX (2016) Potential of MR histogram analyses for prediction of response to chemotherapy in patients with colorectal hepatic metastases. Eur Radiol 26:2009–2018
Wang HY, Su ZH, Xu X et al (2016) Dynamic contrast-enhanced MR imaging in renal cell carcinoma: Reproducibility of histogram analysis on pharmacokinetic parameters. Sci Rep 6:29146
Paz MF, Yaya-Tur R, Rojas-Marcos I et al (2004) CpG island hypermethylation of the DNA repair enzyme methyltransferase predicts response to temozolomide in primary gliomas. Clin Cancer Res 10:4933–4938
Ahluwalia MS (2011) American Society of Clinical Oncology 2011 CNS tumors update. Expert Rev Anticancer Ther 11:1495–1497
Reifenberger G, Hentschel B, Felsberg J et al (2012) Predictive impact of MGMT promoter methylation in glioblastoma of the elderly. Int J Cancer 131:1342–1350
Muldoon LL, Gahramanov S, Li X, Marshall DJ, Kraemer DF, Neuwelt EA (2011) Dynamic magnetic resonance imaging assessment of vascular targeting agent effects in rat intracerebral tumor models. Neuro Oncol 13:51–60
Baur M, Preusser M, Piribauer M et al (2010) Frequent MGMT (0(6)-methylguanine-DNA methyltransferase) hypermethylation in long-term survivors of glioblastoma: a single institution experience. Radiol Oncol 44:113–120
Kirk P, He T, Anderson LJ et al (2010) International reproducibility of single breathhold T2* MR for cardiac and liver iron assessment among five thalassemia centers. J Magn Reson Imaging 32:315–319
Pope WB, Lai A, Mehta R et al (2011) Apparent diffusion coefficient histogram analysis stratifies progression-free survival in newly diagnosed bevacizumab-treated glioblastoma. AJNR Am J Neuroradiol 32:882–889
Romano A, Calabria LF, Tavanti F et al (2013) Apparent diffusion coefficient obtained by magnetic resonance imaging as a prognostic marker in glioblastomas: correlation with MGMT promoter methylation status. Eur Radiol 23:513–520
Yan K, Fu Z, Yang C et al (2015) Assessing amide proton transfer (APT) MRI contrast origins in 9L gliosarcoma in the rat brain using proteomic analysis. Mol Imaging Biol 17:479–487
Zaiss M, Schmitt B, Bachert P (2011) Quantitative separation of CEST effect from magnetization transfer and spillover effects by Lorentzian-line-fit analysis of z-spectra. J Magn Reson 211:149–155
Jin T, Wang P, Zong X, Kim S-G (2013) MR imaging of the amide-proton transfer effect and the pH-insensitive nuclear overhauser effect at 9.4 T. Magn Reson Med 69:760–770
Zu Z, Janve VA, Xu J, Does MD, Gore JC, Gochberg DF (2013) A new method for detecting exchanging amide protons using chemical exchange rotation transfer. Magn Reson Med 69:637–647
Lee JS, Xia D, Ge Y, Jerschow A, Regatte RR (2014) Concurrent saturation transfer contrast in in vivo brain by a uniform magnetization transfer MRI. Neuroimage 95:22–28
Zaiss M, Windschuh J, Paech D et al (2015) Relaxation-compensated CEST-MRI of the human brain at 7 T: Unbiased insight into NOE and amide signal changes in human glioblastoma. Neuroimage 112:180–188
Zaiss M, Windschuh J, Goerke S et al (2017) Downfield-NOE-suppressed amide-CEST-MRI at 7 Tesla provides a unique contrast in human glioblastoma. Magn Reson Med 77:196–208
Heo HY, Zhang Y, Jiang S, Lee DH, Zhou J (2016) Quantitative assessment of amide proton transfer (APT) and nuclear overhauser enhancement (NOE) imaging with extrapolated semisolid magnetization transfer reference (EMR) signals: II. Comparison of three EMR models and application to human brain glioma at 3 Tesla. Magn Reson Med 75:1630–1639
Lee DH, Heo HY, Zhang K et al (2017) Quantitative assessment of the effects of water proton concentration and water T1 changes on amide proton transfer (APT) and nuclear overhauser enhancement (NOE) MRI: The origin of the APT imaging signal in brain tumor. Magn Reson Med 77:855–863
Acknowledgements
The authors thank Ms. Mary McAllister for editorial assistance.
Funding
This study was partially supported by grants from National Natural Science Foundation of China (81171322), Guangdong Provincial Natural Science Foundation (2014A030313271, S2012010009114), Guangdong Provincial Science and Technology Project (2014A020212726), Southern Medical University clinical research project (LC2016ZD028), and the National Institutes of Health (R01EB009731, R01CA166171).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Zhibo Wen, MD, PhD.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (Dr. Fangyao Chen) has significant statistical expertise.
No complex statistical methods were necessary for this paper.
Ethical approval
Institutional Review Board approval was obtained.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Study subjects or cohorts overlap
Three study subjects have been previously reported in one of our previous papers, in which we evaluated the diagnostic values of APTw imaging in differentiate PCNSL and malignant gliomas, see Ref. [29].
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Rights and permissions
About this article
Cite this article
Jiang, S., Rui, Q., Wang, Y. et al. Discriminating MGMT promoter methylation status in patients with glioblastoma employing amide proton transfer-weighted MRI metrics. Eur Radiol 28, 2115–2123 (2018). https://doi.org/10.1007/s00330-017-5182-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5182-4