Abstract
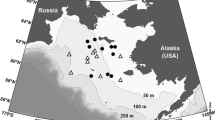
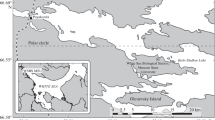
Biological characteristics of ice-associated algal communities were studied in Darnley Bay (western Canadian Arctic) during a 2-week period in July 2008 when the landfast ice cover had reached an advanced stage of melt. We found two distinct and separate algal communities: (1) an interior ice community confined to brine channel networks beneath white ice covers; and (2) an ice melt water community in the brackish waters of both surface melt ponds and the layer immediately below the ice cover. Both communities reached maximum chlorophyll a concentrations of about 2.5 mg m−3, but with diatoms dominating the interior ice while flagellates dominated the melt water community. The microflora of each community was diverse, containing both unique and shared algal species, the latter suggesting an initial seeding of the ice melt water by the bottom ice community. Absorption characteristics of the algae indicated the presence of mycosporine-like amino acids (MAAs) and carotenoid pigments as a photoprotective strategy against being confined to high-light near-surface layers. Although likely not contributing substantially to total annual primary production, these ice-associated communities may play an important ecological role in the Arctic marine ecosystem, supplying an accessible and stable food source to higher trophic levels during the period of ice melt.












Similar content being viewed by others
References
Agawin NSR, Duarte CM, Agustí S (2000) Nutrient and temperature control of the contribution of picoplankton to phytoplankton biomass and production. Limnol Oceanogr 45:591–600
Apollonio S (1985) Arctic marine phototrophic systems: functions of sea ice stabilization. Arctic 38:167–173
Azam F, Fenchel T, Field JG, Gray JS, Meyer-Reil LA, Thingstad F (1983) The ecological role of water-column microbes in the sea. Mar Ecol Prog Ser 10:257–263
Belzile C, Brugel S, Nozais C, Gratton Y, Demers S (2008) Variations of the abundance and nucleic acid content of heterotrophic bacteria in Beaufort Shelf waters during winter and spring. J Mar Syst 74:946–956. doi:10.1016/j.jmarsys.2007.12.010
Bérard-Therriault L, Poulin M, Bossé L (1999) Guide d’identification du phytoplancton marin de l’estuaire du Saint-Laurent, incluant également certains protozoaires. Publ Spéc Can Sci Halieut Aquat 128:1–387
Bricaud A, Stramski D (1990) Spectral absorption coefficients of living phytoplankton and nonalgal biogenous matter: a comparison between the Peru upwelling area and the Sargasso Sea. Limnol Oceanogr 35:562–582
Bricaud A, Claustre H, Ras J, Oubelkheir K (2004) Natural variability of phytoplanktonic absorption in oceanic waters: influence of the size structure of algal populations. J Geophys Res 109:C11010. doi:10.1029/2004JC002419
Buck KR, Nielsen TG, Hansen BW, Gastrup-Hansen D, Thomsen HA (1998) Infiltration phyto- and protozooplankton assemblages in the annual sea ice of Disko Island, West Greenland, spring 1996. Polar Biol 20:377–381
Bursa A (1963) Phytoplankton in coastal waters of the Arctic Ocean at Point Barrow, Alaska. Arctic 16:239–262
Chisholm SW (1992) Phytoplankton size. In: Falkowski PG, Woodhead AD (eds) Primary productivity and biogeochemical cycles in the sea. Plenum Press, New York, pp 213–237
Cleveland JS, Weidemann AD (1993) Quantifying absorption by aquatic particles: a multiple scattering correction for glass-fiber filters. Limnol Oceanogr 38:1321–1327
Ehn JK, Mundy CJ, Barber DG, Hop H, Rossnagel A, Stewart J (in press) Impact of horizontal spreading on light propagation in melt pond covered seasonal sea ice in the Canadian Arctic. J Geophys Res. doi:10.1029/2010JC006908
Eicken H, Krouse HR, Kadko D, Perovich DK (2002) Tracer studies of pathways and rates of meltwater transport through Arctic summer sea ice. J Geophys Res 107:C108046. doi:10.1029/2000JC000583
Ewert M, Deming JW (2011) Selective retention in saline ice of extracellular polysaccharides produced by the cold-adapted marine bacterium Colwellia psychrerythraea strain 34H. Ann Glaciol 52:111–117
Garrison DL, Buck KR (1986) Organism losses during ice melting: a serious bias in sea ice community studies. Polar Biol 6:237–239
Golden KM, Eicken H, Heaton AL, Miner J, Pringle DJ, Zhu J (2007) Thermal evolution of permeability and microstructure in sea ice. Geophys Res Lett 34:L16501. doi:10.1029/2007GL030447
Gradinger R (1996) Occurrence of an algal bloom under Arctic pack ice. Mar Ecol Prog Ser 131:301–305
Gradinger R (1999) Vertical fine structure of the biomass and composition of algal communities in Arctic pack ice. Mar Biol 133:745–754
Gradinger R, Lenz J (1989) Picocyanobacteria in the high Arctic. Mar Ecol Prog Ser 52:99–101
Gradinger R, Meiners K, Plumley G, Zhang Q, Bluhm BA (2005) Abundance and composition of the sea-ice meiofauna in off-shore pack ice of the Beaufort Gyre in summer 2002 and 2003. Polar Biol 28:171–181. doi:10.1007/s00300-004-0674-5
Grasshoff K, Kremling K, Ehrhardt M (1999) Methods of seawater analysis, 3rd edn. Wiley-VCH, New York
Holm-Hansen O, Lorenzen CJ, Holmes RW, Strickland JD (1965) Fluorometric determination of chlorophyll. J Cons Int Explor Mer 30:3–15
Hop H, Mundy CJ, Rossnagel AL, Gosselin M, Barber DG (2011) Zooplankton boom and ice amphipod bust below melting sea ice in the Amundsen Gulf, Arctic Canada. Polar Biol. doi:10.1007/s00300-011-0991-4
Horner RA (2002) A taxonomic guide to some common marine phytoplankton. Biopress Limited, Bristol
Horner R, Schrader GC (1982) Relative contribution of ice-algae, phytoplankton, and benthic microalgae to primary production in nearshore regions of the Beaufort Sea. Arctic 35:485–503
Horner R, Ackley SF, Dieckmann GS, Gulliksen B, Hoshiai T, Legendre L, Melnikov IA, Reeburgh WS, Spindler M, Sullivan C (1992) Ecology of sea ice biota 1. Habitat, terminology, and methodology. Polar Biol 12:417–427
Johnsen G, Samset O, Granskog L, Sakshaug E (1994) In vivo absorption characteristics in 10 classes of bloom-forming phytoplankton: taxonomic characteristics and responses to photoadaptation by means of discriminant and HPLC analysis. Mar Ecol Prog Ser 105:149–157
Junge K, Eicken H, Deming JW (2004) Bacterial activity at −2 to −20°C in Arctic wintertime sea ice. Appl Environ Microbiol 70:550–557. doi:10.1128/AEM.70.1.550-557.2004
Karentz D, McEuen FS, Land MC, Dunlap WC (1991) Survey of mycosporine-like amino acid compounds in Antarctic marine organisms: potential protection from ultraviolet exposure. Mar Biol 108:157–166
Kashino Y, Fujimoto K, Akamatsu A, Koike H, Satoh K, Kudoh S (1998) Photosynthetic pigment composition of ice algal and phytoplankton assemblages in early spring in Saroma Ko Lagoon, Hokkaido, Japan. Proc NIPR Symp Polar Biol 11:22–32
Kirk JTO (1994) Light and photosynthesis in aquatic ecosystems, 2nd edn. Cambridge University Press, Cambridge
Kramer M, Kiko R (2010) Brackish meltponds on Arctic sea ice—a new habitat for marine metazoans. Polar Biol. doi:10.1007/s00300-010-0911-z
Krembs C, Deming JW (2008) The role of exopolymers in microbial adaptation to sea ice. In: Margesin R, Schinner F, Marx JC, Gerday C (eds) Psychrophiles: from biodiversity to biotechnology. Springer, New York, pp 247–264
Krembs C, Mock T, Gradinger R (2001) A mesocosm study on physical-biological interactions in artificial Arctic sea ice. Polar Biol 24:356–364
Krembs C, Eicken H, Junge K, Deming JW (2002) High concentrations of exopolymeric substances in Arctic winter sea ice: implications for the polar ocean carbon cycle and cryoprotection of diatoms. Deep Sea Res I 49:2163–2181. doi:10.1016/S0967-0637(02)00122-X
Krembs C, Eicken H, Deming JW (2011) Exopolymer alteration of physical properties of sea ice and implications for ice habitability and biogeochemistry in a warmer Arctic. Proc Natl Acad Sci USA. doi:10.1073/pnas.1100701108
Kudoh S, Imura S, Kashino Y (2003) Xanthophyll-cycle of ice algae on the sea ice bottom in Saroma Ko lagoon, Hokkaido, Japan. Polar Biosci 16:86–97
Laurion I, Blouin F, Roy S (2003) The quantitative filter technique for measuring phytoplankton absorption: interference by MAAs in the UV waveband. Limnol Oceanogr Methods 1:1–9
Laurion I, Blouin F, Roy S (2004) Packaging of mycosporine-like amino acids in dinoflagellates. Mar Ecol Prog Ser 279:297–303
Leu E, Wiktor J, Søreide JE, Berge J, Falk-Petersen S (2010) Increased irradiance reduces food quality of sea ice algae. Mar Ecol Prog Ser 411:49–60. doi:10.3354/meps08647
Lund JWG, Kipling C, Le Cren ED (1958) The inverted microscope method of estimating algal number and the statistical basis of estimations by counting. Hydrobiologia 11:143–170
Manes SS, Gradinger R (2009) Small scale vertical gradients of Arctic ice algal photophysiological properties. Photosynth Res 102:53–66. doi:10.1007/s11120-009-9489-0
Marie D, Simon N, Vaulot D (2005) Phytoplankton cell counting by flow cytometry. In: Andersen RA (ed) Algal culturing techniques. Academic Press, London, pp 253–268
Maslanik JA, Fowler C, Stroeve J, Drobot S, Zwally J, Yi D, Emery W (2007) A younger, thinner Arctic ice cover: increased potential for rapid extensive sea-ice loss. Geophys Res Lett 34:L24501. doi:10.1029/2007GL0320432007
Medlin LK, Hasle GR (1990) Some Nitzschia and related diatom species from fast ice samples in the Arctic and Antarctic. Polar Biol 10:451–479
Meiners K, Fehling J, Granskog MA, Spindler M (2002) Abundance, biomass and composition of biota in Baltic sea ice and underlying water (March 2000). Polar Biol 25:761–770. doi:10.1007/s00300-002-0403-x
Meiners K, Gradinger R, Fehling J, Civitarese G, Spindler M (2003) Vertical distribution of exopolymer particles in sea ice of the Fram Strait (Arctic) during autumn. Mar Ecol Progr Ser 248:1–13
Meiners K, Krembs C, Gradinger R (2008) Exopolymer particles: microbial hotspots of enhanced bacterial activity in Arctic fast ice (Chukchi Sea). Aquat Microb Ecol 52:195–207. doi:10.3354/ame01214
Melnikov IA, Kolosova EG, Welch HE, Zhitina LS (2002) Sea ice biological communities and nutrient dynamics in the Canada Basin of the Arctic Ocean. Deep Sea Res I 49:1623–1649
Michel C, Legendre L, Therriault J-C, Demers S, Vandevelde T (1993) Springtime coupling between ice algal and phytoplankton assemblages in Southeastern Hudson Bay, Canadian Arctic. Polar Biol 13:441–449
Mundy CJ, Gosselin M, Ehn JK, Gratton Y, Rossnagel AL, Barber DG, Martin J, Tremblay J-É, Palmer M, Arrigo K, Darnis G, Fortier L, Else B, Papakyriakou TN (2009) Contribution of under-ice primary production to an ice-edge upwelling phytoplankton bloom in the Canadian Beaufort Sea. Geophys Res Lett 36:L17601. doi:10.1029/2009GL038837
Parsons TR, Maita Y, Lali CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, Toronto
Perovich DK (2005) On the aggregate-scale partitioning of solar radiation in Arctic sea ice during the Surface Heat Budget of the Artic Ocean (SHEBA) field experiment. J Geophy Res 110:C03002. doi:10.1029/2004JC002512
Perovich DK (2006) The interaction of ultraviolet light with Arctic sea ice during SHEBA. Ann Glaciol 44:47–52
Petrich C, Eicken H (2010) Chapter 2. Growth, structure and properties of sea ice. In: Thomas DN, Dieckmann GS (eds) Sea ice, 2nd edn. Wiley Blackwell Publishing, Oxford. doi:10.1002/9781444317145.ch2
Poulin M (1990) Ice diatoms: the Arctic. In: Medlin LK, Priddle J (eds) Polar marine diatoms. British Antarctic Survey, Cambridge, pp 15–18
Poulin M (1991) Sea ice diatoms (Bacillariophyceae) of the Canadian Arctic. 2. A taxonomic, morphological and geographical study of Gyrosigma concilians. Nord J Bot 10:681–688
Poulin M, Cardinal A (1982) Sea ice diatoms from Manitounuk Sound, southeastern Hudson Bay (Quebec, Canada). II. Naviculaceae, genus Navicula. Can J Bot 60:2825–2845
Pringle DJ, Miner JE, Eicken H, Golden KM (2009) Pore space percolation in sea ice single crystals. J Geophys Res 114:C12017. doi:10.1029/2008JC005145
Rex M, Salawitch RJ, von der Gathen P, Harris NRP, Chipperfield MP, Naujokat B (2004) Arctic ozone loss and climate change. Geophys Res Lett 31:L04116. doi:10.1029/2003GL018844
Riedel A, Michel C, Gosselin M, LeBlanc B (2008) Winter–spring dynamics in sea-ice carbon cycling in the coastal Arctic Ocean. J Mar Syst 74:918–932. doi:10.1016/j.jmarsys.2008.01.003
Robineau B, Legendre L, Therriault J-C, Fortier L, Rosenberg G, Demers S (1994) Ultra-algae (5 μm) in the ice, at the ice-water interface and in the under-ice water column (southeastern Hudson Bay, Canada). Mar Ecol Prog Ser 115:169–180
Robineau B, Legendre L, Michel C, Budeus G, Kattner G, Schneider W, Pesant S (1999) Ultraphytoplankton abundances and chlorophyll a concentrations in ice-covered waters of northern seas. J Plankton Res 21:735–755
Roy S (2000) Strategies for the minimisation of UV-induced damage. In: de Mora SJ, Demers S, Vernet M (eds) The effects of UV radiation in the marine environment. Cambridge University Press, Cambridge, pp 177–205
Różańska M, Gosselin M, Poulin M, Wiktor JM, Michel C (2009) Influence of environmental factors on the development of bottom ice protist communities during the winter–spring transition. Mar Ecol Prog Ser 386:43–59. doi:10.3354/meps08092
Siefermann-Harms D (1987) The light-harvesting and protective functions of carotenoids in photosynthetic membranes. Physiol Plantarium 69:561–568
Smith REH, Anning J, Clement P, Cota G (1988) Abundance and production of ice algae in Resolute Passage, Canadian Arctic. Mar Ecol Prog Ser 48:251–263
Smith REH, Clement P, Cota G (1989) Population dynamics of bacteria in Arctic sea ice. Microb Ecol 17:63–76
Smith REH, Harrison WG, Harris LR, Herman AW (1990) Vertical fine structure of particulate matter and nutrients in sea ice of the high Arctic. Can J Fish Aquat Sci 47:1348–1355
Sosik HM (1999) Storage of marine particulate samples for light-absorption measurements. Limnol Oceanogr 44:1139–1141
Stroeve J, Markus T, Meier WN, Miller J (2006) Recent changes in the Arctic melt season. Ann Glaciol 44:367–374. doi:10.3189/172756406781811583
Stuart V, Sathyendranath S, Head EJH, Platt T, Irwin B, Maass H (2000) Bio-optical characteristics of diatom and prymnesiophyte populations in the Labrador Sea. Mar Ecol 201:91–106
Syvertsen EE (1991) Ice algae in the Barents Sea: types of assemblages, origin, fate and role in the ice-edge phytoplankton bloom. Polar Res 10:277–287
Throndsen J, Hasle GR, Tangen K (2007) Phytoplankton of Norwegian coastal waters. Almater Forlag AS, Oslo
Tomas CR (1997) Identifying marine phytoplankton. Academic Press, San Diego
Tremblay J-É, Simpson K, Martin J, Miller L, Gratton Y, Barber D, Price NM (2008) Vertical stability and the annual dynamics of nutrients and chlorophyll fluorescence in the coastal, southeast Beaufort Sea. J Geophys Res 113:C07S90. doi:10.1029/2007JC004547
Tremblay G, Belzile C, Gosselin M, Poulin M, Roy S, Tremblay J-É (2009) Late summer phytoplankton distribution along a 3,500 km transect in Canadian Arctic waters: strong numerical dominance by picoeukaryotes. Aquat Microb Ecol 54:55–70
Uusikivi J, Vähätalo AV, Granskog MA, Sommaruga R (2010) Contribution of mycosporine-like amino acids and colored dissolved and particulate matter to sea ice optical properties and ultraviolet attenuation. Limnol Oceanogr 55:703–713
von Quillfeldt CH (2000) Common diatom species in Arctic spring blooms: their distribution and abundance. Bot Mar 43:499–516
von Quillfeldt CH (2001) Identification of some easily confused common diatom species in Arctic spring blooms. Bot Mar 44:375–389
Acknowledgments
This work is a contribution to the International Polar Year–Circumpolar Flaw Lead system study (IPY–CFL 2008), supported through grants from the Canadian IPY Federal program office, the Natural Sciences and Engineering Research Council and numerous international collaborators. Postdoctoral fellowship support was provided from the Fonds québécois de la recherche sur la nature et les technologies (FQRNT) to C.J.M. and the Centre National d’Études Spatiales (CNES) to J.K.E. Additional funding was provided from the Canadian Museum of Nature to M.P. The participation of H.H. was jointly facilitated by ARCTOS and ArcticNet, within the IPY-project PanAME funded by the Research Council of Norway. We would like to extend our gratitude to Dr. J.-É. Tremblay’s lab for processing nutrient samples, A. Rossnagel for CTD data and assistance in the field, and to B. Philippe, M. Palmer, A. Sallon, T. Brown, J. Ferland, S. Pineault, J. Gagnon, J. Martin, D. Nguyen, R. Maranger, and the officers and crew of the CCGS Amundsen for logistical and postprocessing support. We gratefully acknowledge K. Meiners and two anonymous reviewers for comments that improved this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article belongs to the special issue “Circumpolar Flaw Lead Study (CFL)”, coordinated by J. Deming and L. Fortier.
Appendix
Appendix
See Table 1.
Rights and permissions
About this article
Cite this article
Mundy, C.J., Gosselin, M., Ehn, J.K. et al. Characteristics of two distinct high-light acclimated algal communities during advanced stages of sea ice melt. Polar Biol 34, 1869–1886 (2011). https://doi.org/10.1007/s00300-011-0998-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-011-0998-x