Abstract
Key message
OG1 is involved in JA-regulated anthesis by modulating carbohydrate transport of lodicules in rice.
Abstract
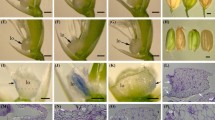
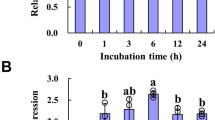
Flowering plants have evolved a sophisticated regulatory network to coordinate anthesis and maximize reproductive success. In addition to various environmental conditions, the plant hormone jasmonic acid and its derivatives (JAs) are involved in anthesis. However, the underlying mechanism remains largely unexplored. Here, we report a JA-defective mutant in rice (Oryza sativa), namely open glume 1, which has dysfunctional lodicules that lead to open glumes following anthesis. Map-based cloning and subsequent complementation tests confirmed that OG1 encodes a peroxisome-localized 12-oxo-phytodienoic acid reductase—a key enzyme that reduces the precursor of JA. Loss-of-function of OG1 resulted in almost no JA accumulation. Exogenous JA treatment completely rescued the defects caused by the og1 mutation. Further studies revealed that intracellular metabolism was disrupted in the lodicules of og1 mutant. At the mature plant stage, most seeds of the mutant were malformed with significantly reduced starch content. We speculate that JA or JA signaling mediates the carbohydrate transport of lodicules during anthesis, and signal the onset of cell degradation in lodicules after anthesis. We conclude that the OPEN GLUME 1 gene that produces a key enzyme involved in reducing the precursor of JA in JA biosynthesis and is involved in carbohydrate transport underlying normal lodicule function during anthesis in rice.









Similar content being viewed by others
Abbreviations
- JA:
-
Jasmonic acid
- OPDA:
-
12-oxo-phytodienoic acid
- OPC 8:0:
-
3-oxo-2-(2′-pentenyl)-cyclopentane-1-octanoic acid
- OPR:
-
12-oxo-phytodienoic acid reductase
References
Acosta IF, Laparra H, Romero SP, Schmelz E, Hamberg M, Mottinger JP, Moreno MA, Dellaporta SL (2009) tasselseed1 is a lipoxygenase affecting jasmonic acid signaling in sex determination of maize. Science 323:262–265
Agrawal GK, Jwa N-S, Shibato J, Han O, Iwahashi H, Rakwal R (2003) Diverse environmental cues transiently regulate OsOPR1 of the “octadecanoid pathway” revealing its importance in rice defense/stress and development. Biochem Biophys Res Commun 310:1073–1082
Avanci NC, Luche DD, Goldman GH, Goldman MHS (2010) Jasmonates are phytohormones with multiple functions, including plant defense and reproduction. Genet Mol Res 9:484–505
Bieleski (1993) Fructan hydrolysis drives petal expansion in the ephemeral daylily flower. Plant Physiol 103, 213–219
Biesgen C, Weiler EW (1999) Structure and regulation of OPR1 and OPR2, two closely related genes encoding 12-oxophytodienoic acid-10,11-reductases from Arabidopsis thaliana. Planta 208:155–165
Biswas KK, Neumann R, Haga K, Yatoh O, Iino M (2003) Photomorphogenesis of rice seedlings: a mutant impaired in phytochrome-mediated inhibition of coleoptile growth. Plant Cell Physiology 44:242–254
Browse J (2009) Jasmonate: preventing the maize tassel from getting in touch with his feminine side. Sci Signal 59:pe9
Buchanan-Wollaston V, Page T, Harrison E et al (2005) Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J 42:567–585
Cai Q, Yuan Z, Chen M et al (2014) Jasmonic acid regulates spikelet development in rice. Nat Commun 5:3476
Caldelari D, Wang G, Farmer EE, Dong X (2011) Arabidopsis lox3 lox4 double mutants are male sterile and defective in global proliferative arrest. Plant Mol Biol 75:25–33
Chandler JW (2010) The hormonal regulation of flower development. J Plant Growth Regul 30:242–254
Chen S, Tao L, Zeng L, Vega-Sanchez ME, Umemura K, Wang GL (2006) A highly efficient transient protoplast system for analyzing defence gene expression and protein–protein interactions in rice. Mol Plant Pathol 7:417–427
Evans RY, Reid MS (1988) Changes in carbohydrates and osmotic potential during rhythmic expansion of rose petals. J Am Soc Hortic Sci 113:884–888
Falkenstein E, Groth B, Mithofer A, Weller E (1991) Methyl jasmonate and a-linolenic acid are potent inducers of tendril coiling. Planta 185:316–322
Feys B, Benedetti CE, Penfold CN, Turner JG (1994) Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6:751–759
Fu JH, Chu JF, Sun XH, Wang J, Yan CY (2012) Simple, rapid, and simultaneous assay of multiple carboxyl containing phytohormones in wounded tomatoes by UPLC-MS/MS using single SPE purification and isotope dilution. Anal Sci 28:1081–1087
Gould SJ, Keller GA, Subramani S (1988) Identification of peroxisomal targeting signals located at the carboxy terminus of four peroxisomal proteins. J Cell Biol 107:897–905
Guo Y, Gan SS (2012) Convergence and divergence in gene expression profiles induced by leaf senescence and 27 senescence-promoting hormonal, pathological and environmental stress treatments. Plant Cell Environ 35:644–655
Haga K, Iino M (2004) Phytochrome-mediated transcriptional up-regulation of ALLENE OXIDE SYNTHASE in rice seedlings. Plant Cell Physiol 45:119–128
He Y, Fukushige H, Hildebrand DF, Gan S (2002) Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol 128:876–884
He YM, Lin YJ, Zeng XC (2012) Dynamic changes of jasmonic acid biosynthesis in rice florets during natural anthesis. Acta Agronom Sin 38:1891–1899
Heslop-Harrison Y, Heslop-Harrison JSH (1996) Lodicule function and filament extension in the grasses: potassium ion movement and tissue specialization. Ann Bot (Lond) 77:573–582
Ishiguro S, Kawa-Oda A, Ueda J, Nishida I, Okada K (2001) The DEFECTIVE IN ANTHER DEHISCENCE1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 13:2191–2209
Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5:387–405
Jung C, Lyou SH, Yeu S, Kim MA, Rhee S, Kim M, Lee JS, Choi YD, Cheong JJ (2007) Microarray-based screening of jasmonate-responsive genes in Arabidopsis thaliana. Plant Cell Rep 26:1053–1063
Katsir L, Chung HS, Koo AJK, Howe GA (2008) Jasmonate signaling: a conserved mechanism of hormone sensing. Curr Opin Plant Biol 11:428–435
Kuiper D, Ribot S, van Reenen HS, Marissen N (1995) The effect of sucrose on the flower bud opening of ‘Madelon’ cut roses. Sci Hortic 60:325–336
Lee RH, Wang CH, Huang LT, Chen SCG (2001) Leaf senescence in rice plants: cloing and characterization of senescence up-regulated genes. J Exp Bot 52:1117–1121
Li W, Liu B, Yu L, Feng D, Wang H, Wang J (2009) Phylogenetic analysis, structural evolution and functional divergence of the 12-oxo-phytodienoate acid reductase gene family in plants. BMC Evol Biol 9:90
Li W, Zhou F, Liu B, Feng D, He Y, Qi K, Wang H, Wang J (2011) Comparative characterization, expression pattern and function analysis of the 12-oxo-phytodienoic acid reductase gene family in rice. Plant Cell Rep 30:981–995
Li K, Liu S, Tan Y, Chao N, Tian X, Qi L, Powell WA, Jiang X, Gai Y (2013) Optimized GC-MS method to simultaneously quantify acetylated aldose, ketose, and alditol for plant tissues based on derivatization in a methyl sulfoxide/1-methylimidazole system. J Agric Food Chem 61:4011–4018
Liao L, Shi CH, Zeng DD, Jin XL, Wu JG (2014) Morphogenesis and molecular basis on the unclosed glumes, a novel mutation related to the floral organ of rice. Plant Mol Biol Rep 33:480–489
Liu L, Li H, Zeng H, Cai Q, Zhou X, Yin C (2015) Exogenous jasmonic acid and cytokinin antagonistically regulate rice flag leaf senescence by mediating chlorophyll degradation, membrane deterioration, and senescence-associated genes expression. J Plant Growth Regul 35:366–376
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
McConn M, Browse J (1996) The critical requirement for linolenic acid is pollen development, not photosynthesis, in an Arabidopsis mutant. Plant Cell 8:403–416
Nelson BK, Cai X, Nebenfuhr A (2007) A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J 51:1126–1136
Norikoshi R, Imanishi H, Ichimura K (2013) Changes in cell number, osmotic potential and concentrations of carbohydrates and inorganic ions in Tweedia caerulea during flower opening. J Jpn Soc Hortic Sci 82:51–56
Park JH, Halitschke R, Kim HB, Baldwin IT, Feldmann KA, Feyereisen R (2002) A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J 31:1–12
Qin Y, Yang J, Zhao J (2005) Calcium changes and the response to methyl jasmonate in rice lodicules during anthesis. Protoplasma 225:103–112
Reinbothe C, Springer A, Samol I, Reinbothe S (2009) Plant oxylipins: role of jasmonic acid during programmed cell death, defence and leaf senescence. FEBS J 276:4666–4681
Riemann M, Riemann M, Takano M (2008) Rice JASMONATE RESISTANT 1 is involved in phytochrome and jasmonate signalling. Plant Cell Environ 31:783–792
Riemann M, Haga K, Shimizu T et al (2013) Identification of rice Allene Oxide Cyclase mutants and the function of jasmonate for defence against Magnaporthe oryzae. Plant J 74:226–238
Sanders PM, Lee PY, Biesgen C, Boone JD, Beals TP, Weiler EW, Goldberg RB (2000) The Arabidopsis DELAYED DEHISCENCE1 gene encodes an enzyme in the jasmonic acid synthesis pathway. Plant Cell 12:1041–1061
Schaller F (2001) Enzymes of the biosynthesis of octadecanoid-derived signalling molecules. J Exp Bot 52:11–23
Schaller F, Weiler EW (1997) Molecular cloning and characterization of OPDA. J Biol Chem 272:28066–28072
Schaller F, Henning P, Weiler EW (1998) OPDA: occurence of two isoenzymes of different specificity against stereoisomers of OPDA. Plant Physiol 118:1345–1351
Schaller F, Biesgen C, Mussig C, Altmann T, Weiler EM (2000) 12-Oxophytodienoate reductase 3 (OPR3) is the isoenzyme involved in jasmonate biosynthesis. Planta 210:979–984
Sheard LB, Tan X, Mao H et al (2010) Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468:400–405
Sosso D, Luo D, Li QB et al (2015) Seed filling in domesticated maize and rice depends on SWEET-mediated hexose transport. Nat Genet 47:1489–1493
Staswick PE, Tiryaki I (2004) The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16:2117–2127
Stintzi A, Browse J (2000) The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc Natl Acad Sci USA 97:10625–10630
Stitz M, Hartl M, Baldwin IT, Gaquerel E (2014) Jasmonoyl-L-isoleucine coordinates metabolic networks required for anthesis and floral attractant emission in wild tobacco (Nicotiana attenuata). Plant Cell 26:3964–3983
Strabner J, Furholz A, Marcheroux P, Amrhein N, Schaller A (1999) A homolog of old yellow enzyme in tomato. J Biol Chem 274:35067–35073
Tan Y, Li K, Hu L, Chen S, Gai Y, Jiang X (2010) Fast and simple droplet sampling of sap from plant tissues and capillary microextraction of soluble saccharides for picogram-scale quantitative determination with GC-MS. J Agric Food Chem 58:9931–9935
Tani T, Sobajima H, Okada K, Chujo T, Arimura S, Tsutsumi N, Nishimura M, Seto H, Nojiri H, Yamane H (2008) Identification of the OsOPR7 gene encoding 12-oxophytodienoate reductase involved in the biosynthesis of jasmonic acid in rice. Planta 227:517–526
von Malek B, van der Graaff E, Schneitz K, Keller B (2002) The Arabidopsis male-sterile mutant dde2-2 is defective in the ALLENE OXIDE SYNTHASE gene encoding one of the key enzymes of the jasmonic acid biosynthesis pathway. Planta 216:187–192
Wang Z, Gu YJ, Gao YZ (1991) Studies on the mechanism of the anthesis of rice III. structure of the lodicule and changes of its contents during flowering. Acta Agronom Sin 17:96–101
Wasternack C (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot (Lond) 100:681–697
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. an update to the 2007 review in Annals of Botany. Ann Bot (Lond) 111:1021–1058
Xiao Y, Chen Y, Charnikhova T et al (2014) OsJAR1 is required for JA-regulated floret opening and anther dehiscence in rice. Plant Mol Biol 86:19–33
Xie D, Feys BF, James S, Nieto-Rostro M, Turner JG (1998) COI1: an arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280:1091–1094
Yan Y, Christensen S, Isakeit T, Engelberth J, Meeley R, Hayward A, Emery RJ, Kolomiets MV (2012) Disruption of OPR7 and OPR8 reveals the versatile functions of jasmonic acid in maize development and defense. Plant Cell 24:1420–1436
Yap Y-M, Loh C-S, Ong B-L (2008) Regulation of flower development in Dendrobium crumenatum by changes in carbohydrate contents, water status and cell wall metabolism. Sci Hortic 119:59–66
Zeng X, Zhou X, Zhang W, Murofushi N, Kitahara T, Kamuro Y (1999) Opening of rice floret in rapid response to methyl jasmonate. J Plant Growth Regul 18:153–158
Zhai Q, Zhang X, Wu F, Feng H, Deng L, Xu L, Zhang M, Wang Q, Li C (2015) Transcriptional mechanism of jasmonate receptor COI1-mediated delay of flowering time in Arabidopsis. Plant Cell 27:2814–2828
Zhang H, Zhou C (2013) Signal transduction in leaf senescence. Plant Mol Biol 82:539–545
Zhou S, Wang Y, Li W et al (2011) Pollen semi-sterility1 encodes a kinesin-1-like protein important for male meiosis, anther dehiscence, and fertility in rice. Plant Cell 23:111–129
Zhu QH, Ramm K, Shivakkumar R, Dennis ES, Upadhyaya NM (2004) The ANTHER INDEHISCENCE1 gene encoding a single MYB domain protein is involved in anther development in rice. Plant Physiol 135:1514–1525
Acknowledgements
We thank Prof. Xiangning Jiang (Beijing Forestry University) for technical guidance on HPLC-MS, and the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences for measurement of endogenous JA content. We also acknowledge support from the Key Laboratory of Biology, Genetics and Breeding of Japonica Rice in Mid-lower Yangtze River, Ministry of Agriculture, P.R. China, and Jiangsu Collaborative Innovation Center for Modern Crop Production, This research was supported by grants from The National Key Research and Development Program of China (Grant: 2016YFD0101801), 863 National High-tech R&D Program of China (2014AA10A603-15), Jiangsu Science and Technology Development Program (BE2014394), and Jiangsu Agricultural Science and Technology Innovation Fund Project (CX(16)1029).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by Kang Chong.
Xiaohui Li and Yihua Wang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, X., Wang, Y., Duan, E. et al. OPEN GLUME1: a key enzyme reducing the precursor of JA, participates in carbohydrate transport of lodicules during anthesis in rice. Plant Cell Rep 37, 329–346 (2018). https://doi.org/10.1007/s00299-017-2232-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-017-2232-y