Abstract
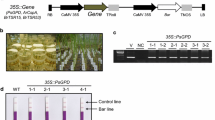
Transgenics for the expression of β-carotene biosynthetic pathway in the endosperm were developed in indica rice background by introducing phytoene synthase (psy) and phytoene desaturase (crtI) genes through Agrobacterium-mediated transformation, employing non-antibiotic positive selectable marker phosphomannose isomerase (pmi). Twenty-seven transgenic lines were characterized for the structural organization of T-DNA inserts and the expression of transgenes in terms of total carotenoid and β-carotene accumulation in the endosperm. Ten lines were also studied for the inheritance of transgenic loci to the T1 progenies. Copy number and sites of integration of the transgenes ranged from one to four. Almost 50% of the transgenic lines showed rearrangement of T-DNA inserts. However, most of the rearrangements occurred in the crtI expression cassette which is adjacent to the right T-DNA border. Differences in copy numbers of psy and crtI were also observed indicating partial T-DNA integration. Beyond T-DNA border transfer was also detected in 25% of the lines. Fifty percent of the lines studied showed single Mendelian locus inheritance, while two lines showed bi-locus inheritance in the T1 progenies. Some of the lines segregating in 3:1 ratio showed two sites of integration on restriction digestion analysis indicating that the T-DNA insertion sites were tightly linked. Three transgenic lines showed nonparental types in the segregating progenies, indicating unstable transgenic locus. Evidences from the HPLC analysis showed that multiple copies of transgenes had a cumulative effect on the accumulation of carotenoid in the endosperm. T1 progenies, in general, accumulated more carotenoids than their respective parents, the highest being 6.77 μg/g of polished seeds. High variation in the carotenoid accumulation was observed within the T1 progenies which could be attributed to the variation in the structural organization and expression of transgenes, minor variations in the genetic background within the progeny plants, or differences in the plant microenvironments. The study identified lines worthy of further multiplication and breeding based on transgene structural integrity in the segregating progeny and high expression levels in terms of the β-carotene accumulation.







Similar content being viewed by others
References
Afolabi AS, Worland B, Snape JW, Vain P (2004) A large-scale study of rice plants transformed with different T-DNAs provides new insights into locus composition and T-DNA linkage configurations. Theor Appl Genet 109:815–826
Aldemita RR, Hodges TK (1996) Agrobacterium tumifaciens-mediated transformation of japonica and indica rice varieties. Planta 199:612–617
Azhakanandam K, Mccabe MS, Power JB, Lowe KC, Cocking EC, Davey MR (2000) T-DNA transfer, integration, expression and inheritance in rice: effects of plant genotype and Agrobacterium super-virulence. J Plant Physiol 157:429–439
Baisakh N, Datta K, Oliva N, Ona I, Rao GJN, Mew TW, Datta SK (2001) Rapid development of homozygous transgenic rice using anther culture harboring rice chitinase gene for enhanced sheath blight resistance. Plant Biotechnol 18:101–108
Cheng M, Fry JE, Pang S, Zhou H, Hironoka CM, Duncan DR, Conner TW, Wan Y (1997) Genetic transformation of wheat mediated by Agrobacterium tumifaciens. Plant Physiol 115:971–980
Christou P, Twyman RM, Fu X, Wegel E, Kohli A, Stoger E (2001) Transgene integration, organization and expression in cereals. In: Khush GS, Brar DS, Hardy B (eds) Rice genetics IV. Proceedings of 4th international rice genetics symposium, 22–27 October 2000. IRRI, Philippines, pp 449–464
Datta K, Oliva N, Torrizo L, Abrigo E, Khush GS, Datta SK (1996) Genetic transformation of indica and japonica rice by Agrobacterium tumifaciens. Rice Genet Newsl 13:136–139
Datta SK, Torrizo LB, Tu J Oliva NP, Datta K (1997) Production and molecular evaluation of transgenic rice plants. IRRI Discuss Pap Ser 21:1–42
Datta K, Baisakh N, Oliva N, Torrizo L, Abrigo E, Tan J, Rai M, Rehana S, Al-Babili S, Beyer P, Potrykus I, Datta SK (2003) Bioengineered ‘golden’ indica rice cultivars with β-carotene accumulation in the endosperm with hygromycin and mannose selection systems. Plant Biotechnol J 1:81–90
Datta K, Rai M, Parkhi V, Oliva N, Tan J, Datta SK (2006) Improved ‘golden’ indica rice and post-transgeneration of metabolic target products of carotenoids (β-carotene) in transgenic elite cultivars (IR64 and BR29). Curr Sci 71:935–939
De Neve M, De Buck S, Jacobs A (1997) T-DNA integration patterns in co-transformed plant cells suggests that T-DNA repeats originate from co-integration of separate T-DNAs. Plant J 11:15–29
Debuck S, Jacobs A, Van Montagu M, Depicker A (1999) The DNA sequences of T-DNA junctions suggest that complex T-DNA loci are formed by a recombination process resembling T-DNA integration. Plant J 20:295–304
Deroles SC, Gardner RC (1988) Analysis of the T-DNA structure in a large number of transgenic petunias generated by Agrobacterium-mediated transformation. Plant Mol Biol 11:365–377
Dong JJ, Kharb P, Teng WM, Hall TC (2001) Characterization of rice transformed via Agrobacterium-mediated inflorescence approach. Mol Breed 7:187–194
Elmayan T, Vaucheret H (1996) Expression of single copies of a strongly expressed 35S transgene can be silenced posttranscriptionally. Plant J 9:787–797
Fu X, Duc LT, Fontana S, Bong BB, Tinjuangjun P, Sudhakar D, Twyman RM, Christou P, Kohli A (2000) Linear transgene constructs lacking vector backbone sequences generate low-copy-number transgenic plants with simple integration patterns. Transgenic Res 9:11–19
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Hoa TT, Al-Babili S, Schaub P, Potrykus I, Beyer P (2003) Golden indica and japonica rice lines amenable to deregulation. Plant Physiol 133:161–169
Hobbs SLA, Warkentin TD, DeLong CMO (1993) Transgene copy number can be positively or negatively associated with transgene tobacco transformants. Plant Mol Biol 15:851–864
Ishida Y, Saito H, Ohta S, Hiei Y, Komari T, Kumashiro T (1996) High efficiency transformation of maize (Zea mays L.) mediated by Agrobacterium tumefaciens. Nat Biotechnol 14:745–750
Jackson SA, Zhang P, Chen WP, Phillips RL, Friebe B, Muthukrishnan S, Gill BS (2001) High resolution structural analysis of biolistic transgene integration into the genome of wheat. Theor Appl Genet 103:56–62
Jorgensen RA, Cluster PD, English J, Que Q, Napoli CA (1996) Chalcone synthase cosuppression phenotypes in petunia flowers: comparison of sense vs. antisense constructs and single copy vs. complex T-DNA sequences. Plant Mol Biol 31:957–973
Kim SR, Lee J, Jun SH, Park S, Kang HG, Kwon S, An G (2003) Transgene structures in T-DNA-inserted rice plants. Plant Mol Biol 52:761–773
Kohli A, Leech MJ, Vain P, Laurie DA, Christou P (1998) Transgene organization in rice engineered through direct DNA transfer supports a two-phase integration mechanism mediated by the establishment of integration hot-spots. Proc Natl Acad Sci USA 95:7203–7208
Kohli A, Gahakwa D, Vain P, Laurie DA, Christou P (1999) Transgene expression in rice engineered through particle bombardment: molecular factors controlling stable expression and transgene silencing. Planta 208:88–97
Kohli A, Twyman RM, Abranches RW, Wegel E, Stoger E, Christou P (2003) Transgene integration organization and interaction in plants. Plant Mol Biol 52:247–258
Kononov ME, Bassuner B, Gelvin SB (1997) Integration of T-DNA binary vector “backbone” sequences into the tobacco genome: evidence for multiple complex patterns of integration. Plant J 11:945–957
Kumar S, Fladung M (2000) Transgene repeats in aspen: molecular characterization suggests simultaneous integration of independent T-DNAs into receptive hotspots in the host genome. Mol Gen Genet 264:20–28
Makarevitch I, Svitashev SK, Somers DA (2003) Complete sequence analysis of transgene loci from plants transformed via microprojectile bombardment. Plant Mol Biol 52:421–432
Martineau B, Voelker TA, Sanders RA (1994) On defining T-DNA. Plant Cell 6:1032–1033
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight DNA. Nucleic Acids Res 8:833–840
Offringa R, deGroot MJA, Haagsman HJ, Does MP, Vandenelzen PJM, Hooykaas PJJ (1990) Extrachromosomal homologous recombination and gene targeting in plant cells after Agrobacterium mediated transformation. EMBO J 9:3077–3084
Paine JA, Shipton CA, Sunandha C, Howells RM, Kennedy MJ, Vermon G, Wright SY, Hinchliffe E, Adams JL, Silverstone AL, Drake R (2005) Improving the nutritional value of golden rice through increased pro-vitamin A content. Nat Biotechnol 23:482–487
Parkhi V, Rai M, Tan J, Oliva N, Rehana S, Bandopadhyay A, Torrizo L, Ghole V, Datta K, Datta SK (2005) Molecular characterization of marker-free transgenic lines of indica rice that accumulate β-carotene in the endosperm. Mol Gen Genomics 274:325–336
Pawlowski WP, Somers DA (1998) Transgenic DNA integrated into the oat genome is frequently interspersed by host DNA. Proc Natl Acad Sci USA 95:12106–12110
Peterhans A, Schlupmann H, Basse C, Paszkowski J (1990) Intrachromosomal recombination in plants. EMBO J 9:3437–3445
Puchta H, Kocher S, Hohn B (1992) Extrachromosomal homologous DNA recombination in plant-cells is fast and is not affected by CpG methylation. Mol Cell Biol 12:3372–3379
Ramanathan V, Veluthambi K (1995) Transfer of non-T-DNA portions of the Agrobacterium tumefaciens Ti plasmid pTiA6 from the left terminus of TL-DNA. Plant Mol Biol 28:1149–1154
Reineri DM, Bottino P, Gordon MP, Nester EW (1990) Agrobacterium-mediated transformation of rice (Oryza sativa L.). BioTechnol 8:33–38
Rodriguez-Amaya DB (2001) A guide to carotenoid analysis in foods. ILSI Press, International Life Science Institute, Washington DC, p 64
Romano E, Soares A, Proite K, Neiva S, Grossi M, Faria JC, Rech EL, Aragao FJL (2005) Transgene elimination in genetically modified dry bean and soybean lines. Genet Mol Res 4:177–184
Sallaud C, Meynard D, Van Boxtel J, Gay C, Bes M, Brizard JP, Larmande P, Ortega D, Raynal M, Portefaix M, Ouwerkerk PBF, Rueb S, Delseny M, Guiderdoni E (2003) Highly efficient production and characterization of T-DNA plants in rice (Oriza sativa L.) functional genomics. Theor Appl Genet 106:1396–1408
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor, New York
Stoger E, Williams S, Keen D, Christou P (1998) Molecular characteristics of transgenic wheat and the effect on transgene expression. Transgenic Res 7:463–471
Svitashev SK, Somers DA (2001) Genomic interspersions determine the size and complexity of transgene loci in transgenic plants produced by microprojectile bombardment. Genome 44:691–697
Tan J, Baisakh N, Oliva N, Parkhi V, Rai M, Torrizo L, Datta K, Datta SK (2005) The screening of rice germplasm, including those transgenic rice lines which accumulate β-carotene in their polished seeds, for their carotenoid profile. Int J Food Sci Tech 40:1–7
Tingay S, McEllroy D, Kalla R, Fieg S, Wang M, Thornton S, Brettell R (1997) Agrobacterium tumefaciens- mediated barley transformation. Plant J 11:1369–1376
Vain P, Afolabi AS, Worland B, Snape JW (2003) Transgene behaviour in populations of rice plants transformed using a new dual binary vector system: pGreen/pSoup. Theor Appl Genet 107:210–217
van der Graff E, den Dulk-Ras A, Hooykas PJJ (1996) deviating T-DNA transfer from Agrobacterium tumefaciens to plants. Plant Mol Biol 31:677–681
Vaucheret H, Beclin C, Elmayan T, Feuerbach F, Godon C, Morel JB, Mourrain P, Palauqui JC, Vernhettes S (1998) Transgene-induced gene silencing in plants. Plant J 16:651–659
Veluthambi K, Gupta AK, Sharma A (2003) The current status of plant transformation technologies. Curr Sci 84:368–380
Yang L, Ding J, Zhang C, Jia J, Weng H, Liu W, Zhang D (2005) Estimating the copy number of transgenes in transformed rice by real-time quantitative PCR. Plant Cell Rep 23:759–763
Ye X, Al-Babili S, Kloti A, Zhang J, Lucca P, Beyer P, Potrykus I (2000) Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid free) rice endosperm. Science 287:303–305
Yin Z, Wang GL (2000) Evidence of multiple complex patterns of T-DNA integration into the rice genome. Theor Appl Genet 100:461–470
Acknowledgments
Financial support from USAID and HarvestPlus is acknowledged. Thanks are due to Syngenta for an international collaborative programme. We thank Dr. Peter Beyer for providing the pCaCar plasmid. The authors are grateful to Ms. Lina Torrizo for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Ebinuma.
Rights and permissions
About this article
Cite this article
Rai, M., Datta, K., Parkhi, V. et al. Variable T-DNA linkage configuration affects inheritance of carotenogenic transgenes and carotenoid accumulation in transgenic indica rice. Plant Cell Rep 26, 1221–1231 (2007). https://doi.org/10.1007/s00299-007-0333-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-007-0333-8