Abstract
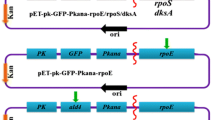
Pyrroloquinoline quinone (PQQ) is a versatile quinone cofactor participating in numerous biological processes. Klebsiella pneumoniae can naturally synthesize PQQ for harboring intact PQQ synthesis genes. Previous metabolic engineering of K. pneumoniae failed to overproduce PQQ due to the employment of strong promoter in expression vector. Here we report that a moderate rather than strong promoter is efficient for PQQ production. To screen an appropriate promoter, a total of four distinct promoters—lac promoter, pk promoter of glycerol dehydratase gene (dhaB1), promoter of kanamycin resistance gene, and T7 promoter (as the control)—were individually used for overexpressing the endogenous PQQ genes in K. pneumoniae along with heterologous expression in Escherichia coli. We found that all recombinant K. pneumoniae strains produced more PQQ than recombinant E. coli strains that carried corresponding vectors, indicating that K. pneumoniae is superior to E. coli for the production of PQQ. Particularly, the recombinant K. pneumoniae recruiting the promoter of kanamycin resistance gene produced the highest PQQ (1,700 nmol), revealing that a moderate rather than strong promoter is efficient for PQQ production. Furthermore, PQQ production was roughly proportional to glucose concentration increasing from 0.5 to 1.5 g/L, implying the synergism between PQQ biosynthesis and glucose utilization. This study not only provides a feasible strategy for production of PQQ in K. pneumoniae, but also reveals the exquisite synchronization among PQQ biosynthesis, glucose metabolism, and cell proliferation.




Similar content being viewed by others
References
Andreeva IG, Golubeva LI, Kuvaeva TM et al (2011) Identification of Pantoea ananatis gene encoding membrane pyrroloquinoline quinone (PQQ)-dependent glucose dehydrogenase and pqqABCDEF operon essential for PQQ biosynthesis. FEMS Microbiol Lett 318(1):55–60
Choi O, Kim J, Kim JG et al (2008) Pyrroloquinoline quinone is a plant growth promotion factor produced by Pseudomonas fluorescens B16. Plant Physiol 146(2):657–668
Choi SY, Khemlani LS, Churchich JE (1992) Brain glutamate decarboxylase and pyrroloquinoline quinone. BioFactors 3(3):191–196
Elias MD, Nakamura S, Migita CT et al (2004) Occurrence of a bound ubiquinone and its function in Escherichia coli membrane-bound quinoprotein glucose dehydrogenase. J Biol Chem 279:3078–3083
Felder M, Gupta A, Verma V et al (2000) The pyrroloquinoline quinone synthesis genes of Gluconobacter oxydans. FEMS Microbiol Lett 193(2):231–236
Goosen N, Huinen RG, van de Putte P (1992) A 24-amino-acid polypeptide is essential for the biosynthesis of the coenzyme pyrrolo-quinoline-quinone. J Bacteriol 174(4):1426–1427
Holscher T, Gorisch H (2006) Knockout and overexpression of pyrroloquinoline quinone biosynthetic genes in Gluconobacter oxydans 621H. J Bacteriol 188(21):7668–7676
Kasahara T, Kato T (2003) Nutritional biochemistry: a new redox-cofactor vitamin for mammals. Nature 422:832
Killgore J, Smidt C, Duich L et al (1989) Nutritional importance of pyrroloquinoline quinone. Science 245(4920):850–852
Kim CH, Han SH, Kim KY et al (2003) Cloning and expression of pyrroloquinoline quinone (PQQ) genes from a phosphate-solubilizing bacterium Enterobacter intermedium. Curr Microbiol 47(6):457–461
Li Y, Su MY, Ge XZ et al (2013) Enhanced aldehyde dehydrogenase activity by regenerating NAD+ in Klebsiella pneumoniae and implications for the glycerol dissimilation pathways. Biotechnol Lett 35(10):1609–1615
Magnusson OT, Toyama H, Saeki M et al (2004) Quinone biogenesis: structure and mechanism of PqqC, the final catalyst in the production of pyrroloquinoline quinone. Proc Natl Acad Sci USA 101(21):7913–7918
Matsushita K, Arents JC, Bader R et al (1997) Escherichia coli is unable to produce pyrroloquinoline quinone (PQQ). Microbiology 143:3149–3156
Meulenberg JJ, Sellink E, Riegman NH et al (1992) Nucleotide sequence and structure of the Klebsiella pneumoniae pqq operon. Mol Gen Genet 232(2):284–294
Miyazaki T, Sugisawa T, Hoshino T (2006) Pyrroloquinoline quinone-dependent dehydrogenases from Ketogulonicigenium vulgare catalyze the direct conversion of L-sorbosone to L-ascorbic acid. Appl Envir Microbiol 72(2):1487–1495
Morris CJ, Biville F, Turlin E et al (1994) Isolation, phenotypic characterization, and complementation analysis of mutants of Methylobacterium extorquens AM1 unable to synthesize pyrroloquinoline quinone and sequences of pqqD, pqqG, and pqqC. J Bacteriol 176(6):1746–1755
Petrov K, Petrova P (2009) High production of 2,3-butanediol from glycerol by Klebsiella pneumoniae G31. Appl Microbiol Biotechnol 84(4):659–665
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Shen YQ, Bonnot F, Imsand EM et al (2012) Distribution and properties of the genes encoding the biosynthesis of the bacterial cofactor, pyrroloquinoline quinone. Biochemistry 51(11):2265–2275
Velterop JS, Sellink E, Meulenberg JJ et al (1995) Synthesis of pyrroloquinoline quinone in vivo and in vitro and detection of an intermediate in the biosynthetic pathway. J Bacteriol 177(17):5088–5098
Wang X, Sa N, Wang FH et al (2013) Engineered constitutive pathway in Klebsiella pneumoniae for 3-hydroxypropionic acid production and implications for decoupling glycerol dissimilation pathways. Curr Microbiol 66(3):293–299
Wettstein C, Mohwald H, Lisdat F (2012) Coupling of pyrroloquinoline quinone dependent glucose dehydrogenase to (cytochrome c/DNA)-multilayer systems on electrodes. Bioelectrochemistry 88:97–102
Xiong XH, Zhao Y, Ge X et al (2011) Production and radioprotective effects of pyrroloquinoline quinone. Int J Mol Sci 12(12):8913–8923
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 21076013, 21276014) and National Basic Research Program of China (973 Program) (2012CB725200).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jiguo Sun and Zengye Han contributed equally to this work.
Rights and permissions
About this article
Cite this article
Sun, J., Han, Z., Ge, X. et al. Distinct Promoters Affect Pyrroloquinoline Quinone Production in Recombinant Escherichia coli and Klebsiella pneumoniae . Curr Microbiol 69, 451–456 (2014). https://doi.org/10.1007/s00284-014-0607-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-014-0607-7