Abstract
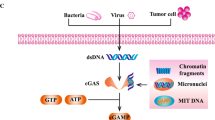
Various cancer therapies, such as surgery, radiotherapy, chemotherapy, and immunotherapy, have been used to treat cancer. Among cancer immunotherapies, stimulators of interferon genes (STING) activate various immune cells and induce them to attack cancer cells. However, the secretion of type I interferon (IFN α and β) increases after stimulation of the immune cell as a side effect of STING agonist, thereby increasing the expression of programmed death-ligand 1 (PD-L1) in the tumor microenvironment (TME). Therefore, it is necessary to reduce the side effects of STING agonists and maximize cancer treatment by administering combination therapy. Tumor-bearing mice were treated with cisplatin, tumor-specific peptide, neoantigen, DMXAA (STING agonist), and immune checkpoint inhibitor (ICI). The combination vaccine group showed a reduction in tumor mass, an increased survival rate, and IFN-γ+ (interferon gamma) CD8+ (cluster of differentiation 8) T cells in the spleen and TME. The distribution of immune cells in the spleen and TME was confirmed, and the number of active immune cells increased, whereas that of immunosuppressive cells decreased. When measuring cytokine levels in the tumor and serum, the levels of pro-inflammatory cytokines increased and anti-inflammatory cytokines decreased. This study demonstrated that when various cancer therapies are combined to treat cancer, it can lead to an anticancer immune synergistic effect by increasing the immune response and reducing side effects.






Similar content being viewed by others
Availability of data and material
The datasets analyzed in the current study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Arruebo M, Vilaboa N, Saez-Gutierrez B, Lambea J, Tres A, Valladares M, Gonzalez-Fernandez A (2011) Assessment of the evolution of cancer treatment therapies. Cancers 3:3279–3330. https://doi.org/10.3390/cancers3033279
Blank CU, Rozeman EA, Fanchi LF et al (2018) Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med 24:1655–1661. https://doi.org/10.1038/s41591-018-0198-0
Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL (2019) Cancer treatment and survivorship statistics, 2019. CA A Cancer J Clin 69:363–385. https://doi.org/10.3322/caac.21565
Richardson JL, Marks G, Levine A (1988) The influence of symptoms of disease and side effects of treatment on compliance with cancer therapy. J Clin Oncol 6:1746–1752. https://doi.org/10.1200/jco.1988.6.11.1746
Vanneman M, Dranoff G (2012) Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 12:237–251. https://doi.org/10.1038/nrc3237
Waldman AD, Fritz JM, Lenardo MJ (2020) A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol 20:651–668. https://doi.org/10.1038/s41577-020-0306-5
Rabinovich GA, Gabrilovich D, Sotomayor EM (2007) Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol 25:267–296. https://doi.org/10.1146/annurev.immunol.25.022106.141609
Park HJ, Jang G-Y, Kim YS et al (2019) A novel TLR4 binding protein, 40S ribosomal protein S3, has potential utility as an adjuvant in a dendritic cell-based vaccine. J Immunother Cancer 7:60. https://doi.org/10.1186/s40425-019-0539-7
Kang TH, Park JH, Yang A et al (2020) Annexin A5 as an immune checkpoint inhibitor and tumor-homing molecule for cancer treatment. Nat Commun 11:1137. https://doi.org/10.1038/s41467-020-14821-z
Lin Y, Xu J, Lan H (2019) Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol 12:76. https://doi.org/10.1186/s13045-019-0760-3
Peng M, Mo Y, Wang Y et al (2019) Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer 18:128. https://doi.org/10.1186/s12943-019-1055-6
Li L, Goedegebuure SP, Gillanders W (2020) Cancer vaccines: shared tumor antigens return to the spotlight. Sig Transduct Target Ther 5:251. https://doi.org/10.1038/s41392-020-00364-8
Hiam-Galvez KJ, Allen BM, Spitzer MH (2021) Systemic immunity in cancer. Nat Rev Cancer. https://doi.org/10.1038/s41568-021-00347-z
Nam G-H, Choi Y, Kim GB, Kim S, Kim SA, Kim I-S (2020) Emerging prospects of exosomes for cancer treatment: from conventional therapy to immunotherapy. Adv Mater 32:2002440. https://doi.org/10.1002/adma.202002440
Budhwani M, Mazzieri R, Dolcetti R (2018) Plasticity of type I interferon-mediated responses in cancer therapy: from anti-tumor immunity to resistance. Front Oncol. https://doi.org/10.3389/fonc.2018.00322
Lu C, Klement JD, Ibrahim ML, Xiao W, Redd PS, Nayak-Kapoor A, Zhou G, Liu K (2019) Type I interferon suppresses tumor growth through activating the STAT3-granzyme B pathway in tumor-infiltrating cytotoxic T lymphocytes. J Immunother Cancer 7:157. https://doi.org/10.1186/s40425-019-0635-8
Aricò E, Castiello L, Capone I, Gabriele L, Belardelli F (2019) Type I interferons and cancer: an evolving story demanding novel clinical applications. Cancers 11:1943
Su T, Zhang Y, Valerie K, Wang X-Y, Lin S, Zhu G (2019) STING activation in cancer immunotherapy. Theranostics 9:7759–7771. https://doi.org/10.7150/thno.37574
Li A, Yi M, Qin S, Song Y, Chu Q, Wu K (2019) Activating cGAS-STING pathway for the optimal effect of cancer immunotherapy. J Hematol Oncol 12:35. https://doi.org/10.1186/s13045-019-0721-x
Hervas-Stubbs S, Perez-Gracia JL, Rouzaut A, Sanmamed MF, Le Bon A, Melero I (2011) Direct effects of type I interferons on cells of the immune system. Clin Cancer Res 17:2619–2627. https://doi.org/10.1158/1078-0432.ccr-10-1114
Zhu Y, An X, Zhang X, Qiao Y, Zheng T, Li X (2019) STING: a master regulator in the cancer-immunity cycle. Mol Cancer 18:152. https://doi.org/10.1186/s12943-019-1087-y
Jiang M, Chen P, Wang L et al (2020) cGAS-STING, an important pathway in cancer immunotherapy. J Hematol Oncol 13:81. https://doi.org/10.1186/s13045-020-00916-z
Barber GN (2015) STING: infection, inflammation and cancer. Nat Rev Immunol 15:760–770. https://doi.org/10.1038/nri3921
Yang H, Lee WS, Kong SJ et al (2019) STING activation reprograms tumor vasculatures and synergizes with VEGFR2 blockade. J Clin Investig 129:4350–4364. https://doi.org/10.1172/JCI125413
Patel SA, Minn AJ (2018) Combination cancer therapy with immune checkpoint blockade: mechanisms and strategies. Immunity 48:417–433. https://doi.org/10.1016/j.immuni.2018.03.007
Yi M, Niu M, Xu L, Luo S, Wu K (2021) Regulation of PD-L1 expression in the tumor microenvironment. J Hematol Oncol 14:10. https://doi.org/10.1186/s13045-020-01027-5
Wang Y, Luo J, Alu A, Han X, Wei Y, Wei X (2020) cGAS-STING pathway in cancer biotherapy. Mol Cancer 19:136. https://doi.org/10.1186/s12943-020-01247-w
Sambi M, Bagheri L, Szewczuk MR (2019) Current challenges in cancer immunotherapy: multimodal approaches to improve efficacy and patient response rates. J Oncol 2019:4508794. https://doi.org/10.1155/2019/4508794
Tang T, Huang X, Zhang G, Hong Z, Bai X, Liang T (2021) Advantages of targeting the tumor immune microenvironment over blocking immune checkpoint in cancer immunotherapy. Sig Transduct Target Ther 6:72. https://doi.org/10.1038/s41392-020-00449-4
Murciano-Goroff YR, Warner AB, Wolchok JD (2020) The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res 30:507–519. https://doi.org/10.1038/s41422-020-0337-2
Kreiter S, Vormehr M, van de Roemer N et al (2015) Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 520:692–696. https://doi.org/10.1038/nature14426
Jiang T, Shi T, Zhang H, Hu J, Song Y, Wei J, Ren S, Zhou C (2019) Tumor neoantigens: from basic research to clinical applications. J Hematol Oncol 12:93. https://doi.org/10.1186/s13045-019-0787-5
Zheng J, Mo J, Zhu T et al (2020) Comprehensive elaboration of the cGAS-STING signaling axis in cancer development and immunotherapy. Mol Cancer 19:133. https://doi.org/10.1186/s12943-020-01250-1
Borst J, Ahrends T, Bąbała N, Melief CJM, Kastenmüller W (2018) CD4+ T cell help in cancer immunology and immunotherapy. Nat Rev Immunol 18:635–647. https://doi.org/10.1038/s41577-018-0044-0
Corrales L, Glickman LH, McWhirter SM et al (2015) Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep 11:1018–1030. https://doi.org/10.1016/j.celrep.2015.04.031
Song L, Yang MC, Knoff J, Wu TC, Hung CF (2014) Cancer immunotherapy employing an innovative strategy to enhance CD4+ T cell help in the tumor microenvironment. PLoS ONE 9:e115711. https://doi.org/10.1371/journal.pone.0115711
Wu CY, Monie A, Pang X, Hung CF, Wu TC (2010) Improving therapeutic HPV peptide-based vaccine potency by enhancing CD4+ T help and dendritic cell activation. J Biomed Sci 17:88. https://doi.org/10.1186/1423-0127-17-88
Hradilova N, Sadilkova L, Palata O, Mysikova D, Mrazkova H, Lischke R, Spisek R, Adkins I (2017) Generation of dendritic cell-based vaccine using high hydrostatic pressure for non-small cell lung cancer immunotherapy. PLoS ONE 12:e0171539. https://doi.org/10.1371/journal.pone.0171539
Lee EJ, Jang G-Y, Lee SE, Jw L, Han HD, Park Y-M, Kang TH (2021) A novel form of immunotherapy using antigen peptides conjugated on PD-L1 antibody. Immunol Lett 240:137–148. https://doi.org/10.1016/j.imlet.2021.10.006
Blass E, Ott PA (2021) Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol 18:215–229. https://doi.org/10.1038/s41571-020-00460-2
Chen F, Zou Z, Du J et al (2019) Neoantigen identification strategies enable personalized immunotherapy in refractory solid tumors. J Clin Investig 129:2056–2070. https://doi.org/10.1172/JCI99538
Yarchoan M, Johnson BA, Lutz ER, Laheru DA, Jaffee EM (2017) Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer 17:209–222. https://doi.org/10.1038/nrc.2016.154
Liu H, Kuang X, Zhang Y et al (2020) ADORA1 inhibition promotes tumor immune evasion by regulating the ATF3-PD-L1 Axis. Cancer Cell 37:324-339.e8. https://doi.org/10.1016/j.ccell.2020.02.006
Herbst RS, Soria J-C, Kowanetz M et al (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515:563–567. https://doi.org/10.1038/nature14011
Acknowledgements
Not applicable.
Funding
This study was supported by National Research Foundation of Korea (NRF) Grants funded by the Korean government (NRF-2016R1A5A2012284 and NRF-2018R1A2B6008455).
Author information
Authors and Affiliations
Contributions
SEL, GYJ, HDH, YMP, and THK designed experiments. SEL, GYJ, JWL, and SHP conducted experiments. SEL and THK analyzed the data and wrote the manuscript. HDH verified the statistical methods used. All the authors provided critical feedback and contributed to the final manuscript. THK supervised this project.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Consent to participate
In the name of all the authors, I gave my full consent to participate.
Consent for publication
I have given my full consent for publication in the name of all authors.
Ethics approval
All animal experiments were approved by the Konkuk University Institutional Animal Care Use Committee (IACUC, KU20104).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, S.E., Jang, GY., Lee, J.w. et al. Improvement of STING-mediated cancer immunotherapy using immune checkpoint inhibitors as a game-changer. Cancer Immunol Immunother 71, 3029–3042 (2022). https://doi.org/10.1007/s00262-022-03220-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03220-6