Abstract
Our progress in understanding pathological disease mechanisms has led to the identification of biomarkers that have had a considerable impact on clinical practice. It is hoped that the move from generalized to stratified approaches, with the grouping of patients into clinical/therapeutic subgroups according to specific biomarkers, will lead to increasingly more effective clinical treatments in the near future. This success depends on the identification of biomarkers that reflect disease evolution and can be used to predict disease state and therapy response, or represent themselves a target for treatment. Biomarkers can be identified by studying relationships between serum, tissue, or tumor microenvironment parameters and clinical or therapeutic parameters at onset and during the progression of the disease, using systems biology. Given that multiple pathways, such as those responsible for redox and immune regulation, are deregulated or altered in tumors, the future of tumor therapy could lie in the simultaneous targeting of these pathways using extracellular and intracellular targets and biomarkers. With this aim in mind, we evaluated the role of thioredoxin 1, a key redox regulator, and CD30, a cell membrane receptor, in immune regulation. Our results lead us to suggest that the combined use of these biomarkers provides more detailed information concerning the multiple pathways affected in disease and hence the possibility of more effective treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The identification of biomarkers has had a considerable impact on clinical practice [1]. As a general rule, if tumor biomarkers are to be of practical use they must either reflect a particular characteristic or stage of disease or represent a target for therapy. One of the best ways to diagnose cancer early, aid in its prognosis, or predict therapeutic response, is to use serum, tissue, or tumor microenvironment biomarkers. Prognostic biomarkers are used to predict the course of the disease, including recurrence and therefore have an important influence on the choice of therapy [2]. Stratification (predictive) biomarkers predict the response to a drug before treatment starts by classifying individuals as likely responders or non-responders. These biomarkers are usually identified from system biology-type studies that look at biological factors that influence not only the pathogenesis but also the progression of the disease. Given that multiple pathways are deregulated or altered in tumors, for example those responsible for redox and immune regulation, it is more than probable that future tumor therapy will involve simultaneous targeting of pathways, through the use of extracellular and intracellular prognostic and stratification biomarkers.
Redox system pathways regulate multiple cellular processes including proliferation, cellular cycle, death, and survival pathways [3–5] and influence the phenotypic, functional, and apoptotic homeostasis of both immunological and tumoral cells. In these pathways, the cell thiol redox state is a crucial mediator of multiple metabolic signaling and transcriptional processes. In a metabolically active cell, these redox system pathways maintain the balance between oxidant and antioxidant factors, by regulating the activation of specific transcription factors and the production of substances that neutralize oxidants [5, 6]. However, when as in tumor, there are alterations in the pathways of the redox system, the cell is no longer able to produce antioxidant substances and adjust the balance between oxidant and antioxidant factors, and therefore, it is unable to respond appropriately to the body’s needs. This is usually the reason why many anticancer agents including radiation are ineffective, because the cytotoxicity induced by them to make the cancer regress affects the antioxidant activity of the redox system pathways, which are altered for the reasons explained above [7]. Alterations in the physiological pathways involved in the regulation of the redox system have been identified in tumors [3–6, 8], and research indicates that the functional inactivation of immune cells by free radicals is an important immunosuppressive mechanism [8]. The re-establishment of homeostasis within the physiological pathways of the redox and immunological system is thus an important therapeutic goal in tumor treatment, and biomarkers and targets are essential for this.
Thioredoxin 1 (Trx1), a thiol-disulfide oxidoreductase, is an essential physiological redox regulator in cellular reactions [6]. As a result of oxidative stress, Trx1 activates specific transcription factors in the nucleus that regulate gene decoding for the production of substances that protect cells from oxidative stress induced by free oxygen radicals. Consequently, the fairly recent discovery that Trx1 catalytically interacts with a target protein, the CD30 cell membrane receptor (CD30R), on immune cells (T, B, monocytes, dendritic cells, NK, eosinophils and granulocytes), is of great clinical significance [9–11]. CD30, although not well known, is a molecule with a wide range of action including regulatory signaling responsible for the physiological homeostasis of T helper (Th)1/Th2/Th3/Th17 cell network functions and hence for a productive immune response [12–16]. The soluble (s) component of CD30 (sCD30) and Trx1 both regulate CD30R functional activation [9, 11], and abnormal increases in the levels of both result in Th1-cell functional deficit [12–14, 17, 18] and have been noted in cancer [19, 20].
In light of the above, we assessed the potential of the combined use of Trx and the CD30 system as a dual biomarker for extracellular and intracellular pathways and propose their simultaneous targeting in tumor redox/immunotherapy.
Trx1 system pathways and their importance in physiological regulation
The redox system is one of the most important regulatory systems of cell physiology in living organisms [6]. The Trx system, consisting of Trx, thioredoxin reductase (TrxR), and NADPH, is a key regulator in cellular redox reactions. The cells of mammals contain two main Trx/TrxR systems: the Trx1/Tx1R system that is normally found in the cytoplasm but under stress can migrate into the nucleus to induce gene transcription or be secreted into the extracellular environment to participate in the immune system network and the Trx2/Trx2R system that is located in mitochondria and endoplasmic reticulum and regulates cellular apoptosis. There is also a mammalian testis-specific thioredoxin system that consists of three thioredoxins that are only expressed in spermatids (named Sptrx-1, Sptrx-2, and Sptrx-3) and an additional thioredoxin, highly expressed in testis, but also present in lung and other ciliated tissues (named Txl-2) [21].
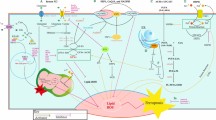
Trx1 consists of 108 amino acids; it contains a disulfide bridge, and its catalytic site (-Trp-Cys32-Gly-Pro-Cys35-Lys) uses hydrogen as a donor for oxidoreduction reactions. Its reduced form is able to reduce ribose or phosphate ribonucleosides, and the oxidized form is converted into the reduced form by the flavoprotein Tx1R, with the intervention of NADPH, forming the “thioredoxin1 redox-system” (Fig. 1). The Trx1 system operates in cellular redox signaling by controlling the activity of transcription factors such as NF-kB, p53, HIFa, AP-1, and the glucocorticoid receptor [6, 18, 22, 23].
Trx1 has a conserved catalytic site (-Trp-Cys-Gly-Pro-Cys-Lys) that undergoes reversible oxidation to the cysteine-disulfide (Trx-S2) form through the transfer of reducing equivalents to a disulfide substrate (X-S2). In health, the oxidized (1) Trx1 form (Trx-S2) is converted back to the cysteine-thiol reduced (2) form [Trx-(SH)2] by the NADPH-dependent flavoprotein thioredoxin reductase (TrxR), so maintaining balance between oxidized and reduced Trx1 forms, and catalytically interacts with CD30 cell membrane receptor (CD30R) on the immune cells (9). The consequent oxidized (3) and reduced (4) states of CD30R determine its respective ability (5) or inability (6) to engage its ligand CD30L. In this way, it is possible to transduce signals of M and DC intracellular pathways for Treg/Th1/Th17 cytokine production, leading to balance of Th differentiation into Treg/Th1/Th17 cells, for immune response homeostasis
Human T cells transformed by viruses produce a factor, previously known as ADF, which is identical to human Trx1. Trx1 is secreted by activated B lymphocytes, B lymphocytes in chronic type-B leukemia, T lymphocytes, and fibroblasts. Trx1 is a potent growth factor and cell survival factor and its expression rises in several types of tumor especially more aggressive ones [24–29], and it is generally related to tumor aggressiveness and inhibition of the immune system [8]. Trx has been evaluated as a biomarker and therapeutic target for cancer [30–32], and it is known that Trx levels can be used to indicate potential chemotherapy resistance [8, 33, 34]. Indeed, an increase in the level of Trx1 has been associated with decreased survival in tumor patients, and it is used as an independent prognostic factor for progression and the expression of VEGF and Ref-1 [29].
Trx1 catalytically interacts (Fig. 1) with a target protein on immune cells, the tumor necrosis factor receptor superfamily member 8 (TNFRSF8/CD30R) [9]. The redox interaction is highly specific for both Trx1 and CD30R, and the consequent redox state of CD30R determines its ability to engage the cognate ligand CD30L and transduce signals [9]. Thus, Trx1 could be used as an extracellular biomarker to reflect CD30-dependent changes in lymphocyte effector function because extracellular Trx levels regulate the redox status of CD30R and binding of its ligand (CD30L) to CD30R (Fig. 1).
The CD30R, CD30L, sCD30, and their importance in immune system homeostasis
The CD30R, a member of the superfamily of TNFR/NGFR receptors, was originally identified on primary cultures of Hodgkin and Sternberg cells. CD30R is also expressed on normal activated T cells, B cells, NK cells, dendritic cells, and macrophages. Activation-induced expression implicates CD30R as one of the regulators of the ensuing immune response. The physiological function of CD30R is not yet clear, but there is evidence that it behaves as a signal transduction molecule. The interaction between CD30R and CD30L on activated T cells, monocytes, neutrophils, eosinophils, and B cells induces rapid activation of gene transcription factors such as JunN-kinase and NFKB. Furthermore, CD30R signals have been shown to induce and regulate the integrated expression of lymphocyte genes, cytotoxic effector molecules, nodal traffic, proliferation, and apoptosis [35]. Under inflammatory conditions, CD30R expression increases and its in vivo activation can be assessed and monitored by measuring the level of sCD30, shed from the plasma membrane upon CD30L binding to CD30R. CD30R was originally identified as a promoter of Th2-cell development and was used as a marker for Th2-cell populations [36, 37]. However, further studies have identified CD30R+ cells in Th0, Th1, and Th2 profiles and shown that during the course of a normal antigen response, CD30R+ cells are the main producers of IL5 and IFNγ [38]. The production of IFNγ by CD30R+ cells has been linked to a TH1 response, while the production of IL4 to a Th2 response. Current research indicates that CD30R and sCD30 levels are involved in the homeostatic regulation of the entire network of Th populations (Th1/Th2/Th3/Th17) [12, 16].
The Th1/Th2/Th3/Th17 cell network and its significance in physiological homeostasis
The immune response has classically been divided into two types: type 1 (Th1) the cell-mediated response, and type 2 (Th2) the humoral immune response. These responses are regulated by Th1 and Th2 subsets of CD4+ Th cells. Both subsets produce interleukin (IL) 3, granulocyte monocyte colony stimulator factor (GMCSF) and tumor necrosis factor (TNF) α, but only Th1 cells produce IL2 and IFNγ, while Th2 cells produce other cytokines such as IL4, IL5, IL6, and IL10. However, for a normal immune system function, the polarization of the immune response into Th1 or Th2 cell types should not be absolute and the ratio of these cells must vary according to physiological demand and clinical conditions [39, 40], and return to initial levels in physiological homeostasis. Each Th cytokine plays a specific and crucial role in maintaining the balance within the Th cell network, which is responsible for the normal development of the immune response.
An additional CD4+ Th-cell subset designated Th3 [41] was later identified, and it is functionally characterized by the production of transforming growth factor (TGF)β. These cells have a very important T-regulatory role (T-reg) on Th1- and Th2-cell functions, and increased effort is being made in pharmacological research to understand the mechanisms behind TGFβ-mediated Th1/Th2 immunological suppression and/or stimulation.
A further population of T cells (Th17 cells) producing IL17, IL6, TNF has also been identified, and it has been reported that TGFβ and IL6 interaction induces Th17-cell differentiation in autoimmunity progression, by switching T-cell generation from Treg to Th17 cells [42, 43]. On the basis of this new Treg/Th1/Th2/Th17 model, Treg and Th17 cells generate from reciprocal developmental pathways, and IL6 and TGFβ appear to be the chief inducers of Th-17 development while TGFβ alone promotes the generation of Treg cells. CD30 appears to regulate the differentiation of Th cells into Treg or Th17 cells [14], and both Th1 and Th2 cytokines are required to reduce IL17.
Consequently, Th1 cells, long thought to mediate tissue damage, might only be involved in the initiation of tissue damage, without playing a decisive role. The cytokine IL17, on the other hand, is now thought to have a major role in various immune-mediated injuries including tumor [44], organ-specific autoimmunity, allergic disorders of the lung and skin, and microbial infections of the intestine and nervous system.
In this new context, the immunological function of Th1-cell population would be to antagonize Th17 pathways to counter the onset of the above-mentioned diseases. Without a doubt, a greater understanding of Treg/Th1/Th17 pathways will lead to a shift in perspective concerning the functional basis of the immune system and lead to improvements in the prevention and treatment of tumor [44] and other immune pathologies. Clearly, given the interconnecting role of the Trx1 and CD30 systems in the regulation of Treg/Th1/Th17 pathways described earlier (Fig. 1), they offer great potential as targets and biomarkers for new pharmacological treatments.
CD30R/sCD30 and their regulatory role in the Treg/Th1/Th17 network
The use of the CD30 system as a biomarker and therapeutic target for cancer (19) has been investigated by various authors [19, 36, 37, 45]. The hypothesis that CD30 system is responsible for the homeostasis of the Treg/Th1/Th17 cell network was confirmed when it was found that CD30R-mediated signals trigger changes in the levels of production of several important Th cytokines involved in the regulation of Treg/Th1/Th17 cell network homeostasis [12–16, 35, 38, 44, 46]. These results also showed that when interaction between CD30R and CD30L was blocked by an abnormal increase in the levels of sCD30 [47], there was an imbalance between Treg/Th1/Th17 cell functions similar to the imbalance described for a monoclonal CD30R antagonist [12] or monoclonal antibodies blocking IL4 or IFNγ activity [12, 46]. The expression and function of the CD30R in dendritic cells (DCs) was also found to have a fundamental role in the production of Th cytokines and regulation of Treg/Th1/Th17 cell network homeostasis [13, 14, 48]; abnormal increases in sCD30 levels produced a disruption of DC CD30R-mediated signals by inducing alterations in the immature DC and DC regulation of Treg/Th1/Th17 cell immune response balance.
Thus, an abnormal increase in the levels of sCD30 can produce an imbalance between Th cell network functions (Fig. 2), by blocking CD30R-mediated signals [47]; sCD30 levels have also been found to be a prediction factor for immunological risks [14, 19, 36, 37]. A lack of Treg can also be a consequence of alterations in the normal regulation of Th cell network through CD30R [12–14]. It has become clear that DCs, through CD30R, play an essential role in inducing and modulating Treg [12–16, 48], and there is increasing evidence that targeted stimulation of Treg may promote health and significantly reduce the risk of cancer [42, 49].
In tumors, an increase in Trx1 levels (1) leads to abnormal redox cellular regulation determining imbalance between oxidant and antioxidant Trx1(2) and between oxidized and reduced states of RCD30 (3), so establishing RCD30 inability to engage CD30L (4) and transducer signals of intracellular pathways for Treg/Th1/Th17 cytokine production (5). When levels of sCD30 (shed from the plasma membrane upon CD30L binding (6) increase (7), sCD30 binds to CD30L with high affinity blocking CD30/CD30L transmembrane signaling (8). Thus, abnormal increases in the levels of Trx1 and sCD30 (biomarkers 9 of extracellular pathways of Trx1/CD30 target) result in both deregulation of M and DC pathways of Treg/Th1/Th17 cytokine production (biomarkers 5 of intracellular pathways of Trx1/CD30 target) and immunological deficit: Th17 expansion and Treg and Th1-cell functional deficit
Hence, in conclusion, it would appear that (i) CD30R-mediated signals are responsible for the homeostasis of important Th cytokines involved in the regulation of Treg/Th1/Th17 cell network homeostasis (Fig. 1 and Online Resource 1); (ii) altered CD30/CD30L interaction due to abnormal increases in sCD30 is responsible for a lack of this regulation and the consequence of this is disease [44] (Fig. 2 and Online Resource 2); (iii) sCD30 can also be used as an extracellular biomarker reflecting CD30R-dependent changes in lymphocyte effector function (Fig. 3) because extracellular sCD30 levels regulate the ligand binding of CD30L to CD30R (Figs. 1, 3).
Levels of Trx1/sCD30 and Treg/Th1/Th17 cytokine in serum, tissue, or tumor microenvironment are prognostic and stratification biomarkers for clinical treatment. The CD30/Trx1 system is a potential target in tumor therapy aimed at the simultaneous optimization of the redox and immune system regulation. Normal levels of sCD30 and Trx1 are positive (benefit) biomarkers reflecting the normal functioning of extracellular pathways, while normal levels of Treg/Th1/Th17 cytokines are positive (benefit) biomarkers for the normal functioning of intracellular pathways. Abnormal levels (higher levels of sCD30, Trx1, and Th17 and lower levels of Treg and Th1) are negative (risk) biomarkers for the abnormal functioning of CD30/Trx1 target pathways and so for immunological deficit and a lack of response to therapy
CD30 and the Trx1 system: their clinical, diagnostic, and therapeutic potential
The pathways mediated by RCD30 are therefore responsible for the homeostasis of the Th cell network, through the regulation of Treg/Th1/Th17 cytokine production by monocytes, immature and mature dendritic cells that direct the differentiation of Th cells (Fig. 1).
However, an abnormal increase in sCD30 levels prevents this regulation by close binding to CD30L, thus blocking its interaction with membrane-bound CD30R [47] (Fig. 2 and Online Resource 2). Hence, sCD30 could potentially have a clinical, diagnostic, and therapeutic role as an extracellular biomarker (Fig. 3) in immunopathologies such as tumors [10, 11].
Trx1, on the other hand, catalytically interacts (Fig. 1) with CD30R [9] determining its ability to engage the cognate ligand CD30L and transduce signals. Thus, Trx1 also affects CD30-dependent changes in lymphocyte effector function by regulating its redox status and ligand binding of CD30L. Increases in Trx1 levels (Fig. 2 and Online Resource 2) have been linked to a lack of this regulation: serum level values of Trx1 (Table 1), which in normal individuals range between 10 and 80 ng/ml (0.8–6.6 nM) [28], have been reported to be elevated in several human primary cancers (Table 1), including lung, colon, cervix, liver, pancreatic, colorectal, and squamous cell cancer [28, 50, 51] (Table 1) and are generally related to tumor aggressiveness [24–29] and inhibition of the immunological system. Serum levels of sCD30 (Table 1), which are usually below detectable levels or present at very low levels between 0 and 10 pg/ml [19, 28], have also been reported to be elevated in several human primary cancers (Table 1), including colorectal, Hodgkin’s lymphoma, prostate, lung and ovarian cancer, and are generally related to tumor aggressiveness [52, 53] and inhibition of the immunological system. Serum levels of sCD30 mirror Trx1 levels in physiological networks [9]: as Trx1 levels increase in infections, allergies, autoimmune events, and cancer, so do sCD30 levels; likewise Trx1 levels modulate CD30R immunological functions during the immune response as do sCD30 levels.
So in light of the facts that (i) RCD30 is highly expressed on immune cells in inflammatory situations and levels of sCD30 and Trx1 increase in the extracellular environment, (ii) changes in the level of sCD30 and Trx1 within normal ranges are linked to physiological immunological homeostasis (Fig. 1 and Online Resource 1), while significantly high levels of sCD30 are related to the prevalence of Th17 suppressive immunity and Treg/Th1 immunological deficiency (Fig. 2 and Online Resource 2), and (iii) higher levels of sCD30 and Trx1 have been found in pathological processes including cancer (Table 1), it can be concluded that (i) the Trx1/CD30 system could represent a new dual target and biomarker in tumor treatments (Fig. 3) and (ii) the levels of Trx1/sCD30 and Treg/Th1/Th17 cytokines could be used as prognostic and stratification biomarkers for extracellular and intracellular pathways in therapy aimed at the simultaneous optimization of the regulation of redox and immune system functions (Fig. 3).
Conclusions
The identification of prognostic and stratification biomarkers in clinical practice has resulted in the transition from a generalized approach to a stratified one, with treatment and diagnosis based on the identification of specific, subgroups of patients. However, this type of approach relies on the definition of real clinical targets and biomarkers which can be easily assessed, early in the disease process.
The potential of Trx and CD30 for use as targets and biomarkers for tumors has been widely described in literature, and alterations in the physiological pathways involved in the regulation of the redox and immunological systems have been identified in various types of tumors such as colorectal cancer, non-small cell lung carcinoma, and breast cancer. However, we believe that it would be more effective to target Trx and CD30 together to optimize the regulation of redox and immune system functions, as by combining these systems we combine the power of redox-sensitive factors with the specificity of immune system molecules (Online Resource 1 and 2), thus opening up important new perspectives in stratified medicine.
In practice, changes in the level of sCD30 in the cellular environment (serum, tissue, or tumor microenvironment) of peripheral blood mononuclear cells, monocytes, and dendritic cells can be used as a biomarker for changes in cytokine production pathways responsible for regulating differentiation of Th cells into Th1, or Th2, or Th3 or Th17 cells (Fig. 1 and Online Resource 1). sCD30 levels within physiological ranges is a positive prognostic biomarker as it reflects homeostasis in the immunological system and therapeutic response. A significant increase in sCD30 levels, on the other hand, is a negative biomarker, indicating immunological deficit and therapeutic risk (Fig. 2). However, both Trx1 and sCD30 can influence the ability of CD30R to mediate the activation of intracellular signaling by selectively inhibiting interaction with CD30L (Fig. 1 and Online Resource 1): Trx1 by catalytically changing RCD30 structure and sCD30 by closely binding to CD30L and blocking its binding to RCD30. Therefore, both components need to be taken into consideration if they are to be effectively used as biomarkers. Changes in levels of Trx1 and sCD30 are the functional extracellular biomarkers of the new Trx1/CD30 target, and the levels of Treg/Th1/Th17 cytokine production are the functional biomarkers of intracellular pathways (Fig. 3). In addition, being able to manipulate their roles in pathological processes may well represent a non-invasive means of redirecting the physiological system toward a beneficial immunological and therapeutic response (Online Resource 2).
In conclusion, these results indicate that the Trx1/CD30 system not only represents a dual prognostic and stratification biomarker for clinical diagnosis and therapeutic risk/benefit indices, but that it also has the potential to be a dual target in translational research into the pharmacological modulation of multiple redox and immunological pathways aimed at the simultaneous optimization of the regulation of redox and immune system functions.
References
Disis ML (2011) Immunologic biomarkers as correlates of clinical response to cancer immunotherapy. Cancer Immunol Immunother 60:433–442
Beachy SH, Repasky EA (2008) Using extracellular biomarkers for monitoring efficacy of therapeutics in cancer patients: an update. Cancer Immunol Immunother 57:759–775
Uno K, Okuno K, Kato T et al (2010) Pre-operative intracellular glutathione levels of peripheral monocytes as a biomarker to predict survival of colorectal cancer patients. Cancer Immunol Immunother 59:1457–1465
Soini Y, Kahlos K, Näpänkangas U et al (2001) Widespread expression of thioredoxin and thioredoxin reductase in non-small cell lung carcinoma. Clin Cancer Res 7:1750–1757
Kakolyris S, Giatromanolaki A, Koukourakis M et al (2001) Thioredoxin expression is associated with lymph node status and prognosis in early operable non-small cell lung cancer. Clin Cancer Res 7:3087–3091
Holmgren A, Lu J (2010) Thioredoxin and thioredoxin reductase: current research with special reference to human disease. Biochem Biophys Res Commun 396:120–124
Malmberg KJ (2004) Effective immunotherapy against cancer: a question of overcoming immune suppression and immune escape? Cancer Immunol Immunother 53:879–892
Kim SJ, Miyoshi Y, Taguchi T et al (2005) High thioredoxin expression is associated with resistance to docetaxel in primary breast cancer. Clin Cancer Res 11:8425–8430
Schwertassek U, Balmer Y, Gutscher M et al (2007) Selective redox regulation of cytokine receptor signaling by extracellular thioredoxin1. EMBO J 26:3086–3097
Van der Vliet HJ, Koon HB, Yue SC et al (2007) Effects of the administration of high-dose interleukin-2 on immunoregulatory cell subsets in patients with advanced melanoma and renal cell cancer. Clin Cancer Res 13:2100–2108
Eichenauer DA, Simhadri VL, von Strandmann EP et al (2007) ADAM10 inhibition of human CD30 shedding increases specificity of targeted immunotherapy in vitro. Cancer Res 67:332–338
Pellegrini P, Berghella AM, Contasta I et al (2003) CD30 antigen: not a physiological marker for TH2 cells but an important costimulator molecule in the regulation of the balance between TH1/TH2 response. Transpl Immunol 12:49–61
Pellegrini P, Totaro R, Contasta I et al (2005) CD30 antigen and multiple sclerosis: CD30, an important costimulatory molecule and marker of a regulatory subpopulation of dendritic cells, is involved in the maintenance of the physiological balance between TH1/TH2 immune responses and tolerance. The role of IFNbeta-1a in the treatment of multiple sclerosis. Neuroimmunomodulation 12:220–234
Contasta I, Totaro R, Berghella AM et al (2010) Soluble CD30: a biomarker for evaluating the clinical risk versus benefit of IFNbeta1A treatment in multiple sclerosis patients. Int J Immunopathol Pharmacol 23:213–226
Zeiser R, Vu-H Nguyen, Hou JZ et al (2007) Early CD30 signaling is critical for adoptively transferred CD4+CD25+ regulatory T cells in prevention of acute graft-versus-host disease. Blood 109:2225–2233
Zeiser R, Vu-H Nguyen, Buess M et al (2005) Prevention of acute graft-versus-host disease by CD4+ CD25+ regulatory T cells is dependent on the CD30/CD153 pathway. Blood 106:1299
Kim SH, Oh J, Choi JY et al (2008) Identification of human thioredoxin as a novel IFN-gamma-induced factor: mechanism of induction and its role in cytokine production. BMC Immunol 9:64
Saraiva M, Smith P, Fallon PG et al (2002) Inhibition of type 1 cytokine-mediated inflammation by a soluble CD30 homologue encoded by ectromelia (mousepox) virus. J Exp Med 196:829–839
Del-Beato T, Berghella AM, Pellegrini P et al (1997) The role of the soluble CD30 serum level in colorectal cancer: a possible marker for a patient subset which could benefit from IL2 biotherapy. Cancer Biother & Radiopharmaceuticals 12:297–304
Kuljaca S, Liu T, Dwarte T et al (2009) The cyclin-dependent kinase inhibitor, p21(WAF1), promotes angiogenesis by repressing gene transcription of thioredoxin-binding protein 2 in cancer cells. Carcinogenesis 30:1865–1871
Miranda-Vizuete A, Sadek CM, Jimenez A et al (2004) The mammalian testis-specific thioredoxin system. Antioxid Redox Signal 6:25–40
Lillig CH, Holmgren A (2007) Thioredoxin and related molecules: from biology to health and disease. Antioxid Redox Signal 9:25–47
Jones DT, Pugh CW, Wigfield S et al (2006) Novel thioredoxin inhibitors paradoxically increase hypoxia-inducible factor-alpha expression but decrease functional transcriptional activity, DNA binding, and degradation. Clin Cancer Res 12:5384–5394
Ceccarelli J, Delfino L, Zappia E et al (2008) The redox state of the lung cancer microenvironment depends on the levels of thioredoxin expressed by tumor cells and affects tumor progression and response to prooxidants. Int J Cancer 123:1770–1778
Lincoln DT, Ali Emadi EM, Tonissen KF et al (2003) The thioredoxin–thioredoxin reductase system: over-expression in human cancer. Anticancer Res 23:2425–2433
Chaiswing L, Bourdeau-Heller JM, Zhong W et al (2007) Characterization of redox state of two human prostate carcinoma cell lines with different degrees of aggressiveness. Free Radic Biol Med 43:202–215
Rubartelli A, Bajetto A, Allavena G et al (1990) Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J Biol Chem 267:24161–24164
Powis G, Mustacichi D, Coon A (2000) The role of the redox protein thioredoxin in cell growth and cancer. Free Radical Biol Med 29:312–322
Welsh SJ, Bellamy WT, Briehl MM et al (2002) The redox protein thioredoxin-1 (Trx-1) increases hypoxia-inducible factor 1alpha protein expression: Trx-1 overexpression results in increased vascular endothelial growth factor production and enhanced tumor angiogenesis. Cancer Res 62:5089–5095
Iwasawa S, Yamano Y, Takiguchi Y et al (2011) Upregulation of thioredoxin reductase 1 in human oral squamous cell carcinoma. Oncol Rep 25:637–644
Lincoln DT, Al-Yatama F, Mohammed FM et al (2010) Thioredoxin and thioredoxin reductase expression in thyroid cancer depends on tumour aggressiveness. Anticancer Res 30:767–775
Selenius M, Rundlöf AK, Olm E et al (2010) Selenium and the selenoprotein thioredoxin reductase in the prevention, treatment and diagnostics of cancer. Antioxid Redox Signal 12:867–880
Thoenes L, Hoehn M, Kashirin R et al (2002) In vivo chemoresistance of prostate cancer in metronomic cyclophosphamide therapy. J Proteomics 73:1342–1354
Nguyen P, Awwad RT, Smart DD et al (2006) Thioredoxin reductase as a novel molecular target for cancer therapy. Cancer Lett 236:164–174
Muta H, Boise LH, Fang L et al (2000) CD30 signals integrate expression of cytotoxic effector molecules, lymphocyte trafficking signals, and signals for proliferation and apoptosis. J Immunol 165:5105–5111
Iwagaki H, Hizuta A, Kohka H et al (1999) Circulating levels of soluble CD30 and other markers in colorectal cancer patients. J Med 30:111–121
Horie R, Watanabe T (1998) CD30: expression and function in health and disease. Semin Immunol 10:457–470
Nakamura T, Lee RK, Nam S et al (1997) Reciprocal regulation of CD30 expression on CD4+ T cells by IL-4 and IFNγ. J Immunol 158:2090–2098
Mosmann TR, Schumacher JH, Street NF et al (1991) Diversity of cytokine synthesis and function of mouse CD4+ T cells. Immunol Rev 123:209–229
Pellegrini P, Berghella AM, Del Beato T et al (1996) Disregulation in TH1 and TH2 subsets of CD4+ T cells in peripheral blood of colorectal cancer patients and involvement in cancer establishment and progression. Cancer Immunol Immunother 42:1–8
Acerwenka A, Swain SL (1999) TGF-β1 immunosuppressant and viability factor for T lymphocytes. Microbes Infect 15:1291–1296
Steinman L (2007) A brief history of TH17, the first major revision in the TH1/TH2 hypothesis of T cell–mediated tissue damage. Nat Med 13:139–144
Bettelli E, Carrier Y, Gao W et al (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235–238
Ji Y, Zhang W (2010) Th17 cells: positive or negative role in tumor? Cancer Immunol Immunother 59(7):979–987
Nishioka C, Takemoto S, Kataoka S et al (2005) Serum level of soluble CD30 correlates with the aggressiveness of adult T-cell leukemia/lymphoma. Cancer Sci 96:810–815
Tang C, Yamada H, Shibata K et al (2008) A novel role of CD30L/CD30 signaling by T–T cell interaction in Th1 response against mycobacterial infection. J Immunol 181:6316–6327
Hargreaves PG, Al-Shamkhani A (2002) A Soluble CD30 blocks transmembrane signaling by CD30. Eur J Immunol 32:163–173
Woodhead VE (1998) From sentinel to messenger: an extended phenotypic analysis of the monocyte to dendritic cells transition. Immunology 94:552–559
Erdman SE, Rao VP, Olipitz W et al (2010) Unifying roles for regulatory T cells and inflammation in cancer. Int J Cancer 126:1651–1665
Grogan TM, Fenoglio-Prieser C, Zeheb R et al (2000) Thioredoxin, a putative oncogene product, is overexpressed in gastric carcinoma and associated with increased proliferation and increased cell survival. Hum Pathol 31:475–481
Raffel J, Bhattacharyya AK, Gallegos A et al (2003) Increased expression of thioredoxin-1 in human colorectal cancer is associated with decreased patient survival. J Lab Clin Med 142:46–51
Nadali G, Tavecchia L, Zanolin E et al (1998) Serum level of the soluble form of the CD30 molecule identifies patients with Hodgkin’s disease at high risk of unfavorable outcome. Blood 91:3011–3016
Zinzani PL, Poleri S, Bendandi M et al (1998) Clinical implications of serum levels of soluble CD30 in 70 adults anaplastic large-cell lymphoma patients. J Clin Oncol 16:1532–1537
Miyazaki K, Noda N, Okada S et al (1998) Elevated serum level of thioredoxin in patients with hepatocellular carcinoma. Biotherapy 11:277–288
Nakamura H, Bai J, Nishinaka Y et al (2000) Expression of thioredoxin and glutaredoxin, redoxregulating proteins, in pancreatic cancer. Cancer Detect Prev 24:53–60
Okamoto M, Azuma K, Hoshino T et al (2009) Correlation of decreased survival and IL-18 in bone metastasis. Intern Med 48:763–773
Baker AF, Dragovich T, Tate WR et al (2006) The antitumor thioredoxin-1 inhibitor PX-12 (1-methylpropyl 2-imidazolyl disulfide) decreases thioredoxin-1 and VEGF levels in cancer patient plasma. J Lab Clin Med 147:83–90
Visco C, Nadali G, Vassilakopoulos TP et al (2006) Very high levels of soluble CD30 recognize the patients with classical Hodgkin's lymphoma retaining a very poor prognosis. Eur J Haematol 77:387
Janik JE, Morris JC, Pittaluga S et al (2004) Elevated serum-soluble interleukin-2 receptor levels in patients with anaplastic large cell lymphoma. Blood 104:3355–3357
Latza U, Foss HD, Dürkop H et al (1995) CD30 antigen in embryonal carcinoma and embryogenesis and release of the soluble molecule. Am J Pathol 146:463–471
Holzer G, Pfandlsteiner T, Blahovec H et al (2003) Serum concentrations of sCD30 and sCD40L in patients with malignant bone tumours. Wien Med Wochenschr 153:40–42
Acknowledgments
We would like to thank the Mayor, Dr. Giuseppe Marulli, and the Deputy Mayor, Mr. Virgilio Lerza, and the administrative staff of Capestrano Town Council (L’Aquila-Italy), for giving us office space following the loss of our building in the earthquake in L’Aquila in 2009.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Berghella, A.M., Pellegrini, P., Del Beato, T. et al. The potential role of thioredoxin 1 and CD30 systems as multiple pathway targets and biomarkers in tumor therapy. Cancer Immunol Immunother 60, 1373–1381 (2011). https://doi.org/10.1007/s00262-011-1068-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-011-1068-5