Abstract
Purpose
The goal of this guideline/procedure standard is to assist nuclear medicine physicians, other nuclear medicine professionals, oncologists or other medical specialists for recommended use of [18F]FDG PET/CT in oncological patients undergoing immunotherapy, with special focus on response assessment in solid tumors.
Methods
In a cooperative effort between the EANM, the SNMMI and the ANZSNM, clinical indications, recommended imaging procedures and reporting standards have been agreed upon and summarized in this joint guideline/procedure standard.
Conclusions
The field of immuno-oncology is rapidly evolving, and this guideline/procedure standard should not be seen as definitive, but rather as a guidance document standardizing the use and interpretation of [18F]FDG PET/CT during immunotherapy. Local variations to this guideline should be taken into consideration.
Preamble
The European Association of Nuclear Medicine (EANM) is a professional non-profit medical association founded in 1985 to facilitate worldwide communication among individuals pursuing clinical and academic excellence in nuclear medicine. The Society of Nuclear Medicine and Molecular Imaging (SNMMI) is an international scientific and professional organization founded in 1954 to promote science, technology and practical application of nuclear medicine. The Australian and New Zealand Society of Nuclear Medicine (ANZSNM), founded in 1969, represents the major professional society fostering the technical and professional development of nuclear medicine practice across Australia and New Zealand. It promotes excellence in the nuclear medicine profession through education, research and a commitment to the highest professional standards. EANM, SNMMI and ANZSNM members are physicians, technologists, physicists and scientists specialized in the research and clinical practice of nuclear medicine. All three societies will periodically put forth new standards/guidelines for nuclear medicine practice to help advance the science of nuclear medicine and improve service to patients. Existing standards/guidelines will be reviewed for revision or renewal, as appropriate, on their fifth anniversary or sooner, if indicated. Each standard/guideline, representing a policy statement by the EANM/SNMMI/ANZSNM, has undergone a thorough consensus process, entailing extensive review. These societies recognize that the safe and effective use of diagnostic nuclear medicine imaging requires particular training and skills, as described in each document. These standards/guidelines are educational tools designed to assist practitioners in providing appropriate and effective nuclear medicine care for patients. These guidelines are consensus documents based on current knowledge. They are not intended to be inflexible rules or requirements of practice, nor should they be used to establish a legal standard of care. For these reasons and those set forth below, the EANM, SNMMI and ANZSNM caution against the use of these standards/guidelines in litigation in which the clinical decisions of a practitioner are called into question. The ultimate judgment regarding the propriety of any specific procedure or course of action must be made by medical professionals considering the unique circumstances of each case. Thus, there is no implication that an action differing from what is laid out in the guidelines/procedure standards, standing alone, is below standard of care. To the contrary, a conscientious practitioner may responsibly adopt a course of action different from that set forth in the standards/guidelines when, in the reasonable judgment of the practitioner, such course of action is indicated by the condition of the patient, limitations of available resources or advances in knowledge or technology subsequent to publication of the guidelines/procedure standards. The practice of medicine involves not only the science, but also the art of dealing with the prevention, diagnosis, alleviation and treatment of disease. The variety and complexity of human conditions make it impossible for general guidelines to consistently allow for an accurate diagnosis to be reached or a particular treatment response to be predicted. Therefore, it should be recognized that adherence to these standards/ guidelines will not ensure a successful outcome. All that should be expected is that practitioners follow a reasonable course of action, based on their level of training, current knowledge, clinical practice guidelines, available resources and the needs/context of the patient being treated. The sole purpose of these guidelines is to assist practitioners in achieving this objective. The present guideline/procedure standard was developed collaboratively by the EANM, the SNMMI and the ANZSNM, with the support of international experts in the field. They summarize also the views of the Oncology and Theranostics and the Inflammation and Infection Committees of the EANM, as well as the procedure standards committee of the SNMMI, and reflect recommendations for which the EANM and SNMMI cannot be held responsible. The recommendations should be taken into the context of good practice of nuclear medicine and do not substitute for national and international legal or regulatory provisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last decade, remarkable achievements in cancer treatment were made with the introduction of the immune checkpoint inhibitors (ICIs) [1, 2]. Most importantly, these have included the development and clinical introduction of antibodies against cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death-associated protein 1 (PD-1) and its ligand 1 (PD-L1). In 2011, ipilimumab, an anti-CTLA-4 monoclonal antibody, was the first to be approved by regulatory authorities based on the significant improvement in overall survival in melanoma [2]. Swiftly, this was followed by the development of monoclonal antibodies targeting PD-1, such as pembrolizumab and nivolumab, as well as those targeting PD-L1, such as avelumab and atezolizumab [3,4,5,6]. These agents act at differing points in T-cell activation with anti-CTLA-4 antibodies affecting early T cell priming and anti-PD-1 and PD-L1 affecting T-cell proliferation and cancer cell killing [7]. They can be used as single- or dual-agent therapies, or in combination with other standard oncological treatments including chemotherapy, radiotherapy or other targeted therapies [8,9,10,11,12]. The rapidly expanded clinical use of ICIs in the treatment of metastatic disease across a broad range of cancers was extended also to adjuvant and neoadjuvant settings in combination with curative intent surgery or radiotherapy of local or regional disease with a high risk of recurrence [12,13,14]. While durable responses can be achieved in a subset of metastatic patients, growing evidence suggests greater efficacy for these agents in the context of low tumor burden, even when conventional imaging does not depict measurable metastatic disease [15]. This is presumably achieved by targeting cancer cells before the development of an increasingly unfavorable tumor microenvironment, which, in turn, results in evolving resistance to immunotherapy treatments [16].
The immense progress of ICIs has brought challenges for cancer management, including a need for the oncological community to reconsider the conventional ways of assessing treatment efficacy and to develop strategies to manage a variety of relatively common immune-related adverse events (irAEs) that are not often encountered with other cancer therapies. It is now recognized that the effectiveness of ICIs is contingent on the successful activation of the multi-step cancer immunity cycle by the host immune cells. This begins with antigen presentation to dendritic cells and subsequently leads to priming, trafficking and infiltration of effector T cells into the tumor microenvironment [17]. Beyond conventional patterns of response as seen in chemotherapy, in a subset of patients unconventional modes of response were noted with delayed apparent efficacy of ICIs. These included a transient increase in tumor burden or even appearance of new lesions, termed “pseudoprogression,” which were attributed to initial recruitment of immune cells at tumor sites [18]. The standard radiographic criteria [19], commonly used to evaluate responses to chemotherapies or targeted therapies, did not account for these new kinetics of response. Hence, several modified evaluation criteria were devised to account for the appearance of new lesions and transient size increase by an extended delay to prove or refute tumor progression. These include immune-related response criteria (irRC) based on bidimensional measurements of target lesions or unidimensional size evaluation such as immune-related RECIST (irRECIST), immune RECIST (iRECIST) and immune-modified RECIST (imRECIST) [18, 20,21,22].
Cancer cells utilize aerobic glycolysis in part to fuel cell growth and energy needs [23], and this represents the fundamental pathway imaged by [18F]FDG PET/CT. Given the complexity of various immunotherapeutic responses, which challenged the conventional framework of RECIST, several studies evaluated the utility of [18F]FDG PET/CT in monitoring the response to ICIs [24,25,26,27,28,29,30,31,32,33]. As with morphological imaging, mechanistic differences in the mode of action of ICIs also challenged extrapolating the success of [18F]FDG PET/CT in monitoring cytotoxic or targeted treatment to evaluate immunotherapeutic strategies with checkpoint inhibitors [34]. Indeed, the very same metabolic reprogramming used by cancer cells extends to T cells, as both cancer cells and tumor infiltrating effector T cells possess high-affinity GLUT1 transporters to facilitate glycolysis [35]. This leads to a complicated interpretation of [18F]FDG PET/CT, especially early (in the first weeks/months) after treatment initiation, since increasing metabolic activity during therapy within the morphologically stable lesions paradoxically may indicate recruitment and activation of immune cells into the tumor microenvironment or draining lymphoid tissues rather than progression. Beyond response monitoring, [18F]FDG PET/CT has shown accuracy in monitoring systemic immune response and detecting irAEs in an early stage. PET/CT-derived quantitative metabolic parameters appeared to have prognostic implications [34, 36,37,38,39]. Therefore, there is an unprecedented need to provide imaging specialists and clinicians with a clinical practice framework for a more accurate, systematic and harmonized interpretation of [18F]FDG PET/CT in the rapidly evolving era of immunotherapeutic strategies.
Goals
The goal of this guideline/procedure standard is to provide nuclear medicine professionals recommendations to correctly perform, interpret and report the results of [18F]FDG PET/CT in oncological patients undergoing immunotherapy, with a special focus on response assessment in solid tumors.
In addition, it provides general information to other professionals and medical specialties related to immune-oncology, i.e., radiologists, medical oncologists and radiation oncologists, for whom the acknowledgment of [18F]FDG PET/CT applications in this clinical context can be useful to support decisions regarding appropriate patient management.
This field is rapidly evolving, and this guideline/procedure standard cannot be seen as definitive, nor is it a summary of all existing protocols. Local variations to this guideline should be taken into consideration within a multidisciplinary setting.
Definitions
Beyond the conventional imaging patterns of tumor response, such as complete/partial response and stable disease, ICIs have been associated with novel atypical patterns of response to treatment [40] that are not, or rarely, observed with conventional cytotoxic or targeted anticancer treatments.
Pseudoprogressive disease
Historically, progressive disease is defined by an increase in size of target or nontarget lesions or by the appearance of new lesions (Fig. 1a). However, a series of clinical trials evaluating the efficacy of ipilimumab in melanoma demonstrated an unconventional pattern of tumor response on conventional imaging with transitory anatomical “progression” followed by response [41,42,43]. This pattern of response was named pseudoprogressive disease (PPD), and new response criteria were created [44, 45]. Most frequently, PPD occurs within the first 4–6 weeks of treatment, but can also occur up to several months after ICI initiation. The rate of PPD varies between tumor types and immunotherapies and has been reported in up to 10% of patients based on CT scan [46, 47] or [18F]FDG PET [48], appearing to be most common in patients with metastatic melanoma treated with anti-CTLA-4 antibody [46, 47], especially with combined ICIs. The PPD phenomenon has been attributed to a variety of mechanisms, including a delayed activation of immune response, local edema due to inflammatory processes and infiltration of immune cells within tumor lesions [49].
Dissociated response
Dissociated response (DR), also known as mixed response or disproportional response (Fig. 1c), is defined by a decrease or stabilization in some tumor sites with a concomitant increase in other sites [50]. In patients treated with ICIs, DR has been reported in up to 10% of the cases [47, 48, 51]. A proportion of patients in retrospective cohorts benefit from prolonging ICI after a dissociated response, despite its classification as true progression by conventional response criteria. Some studies have demonstrated that a dissociated response is associated with a better prognosis than homogeneous progression of lesions in patients treated with ICI [48, 52]. This pattern of response may reflect the heterogeneity of tissue-specific tumor microenvironments, and can be easily detected by [18F]FDG PET due to its high sensitivity and capacity to assess early response on a lesion-by-lesion basis. From a clinical perspective, patients with dissociated response may benefit from treatment beyond progression potentially by continuing checkpoint inhibitor therapy and integrating local treatments, such as surgery, radiotherapy or interventional radiological treatment of oligoprogressive lesions [53].
Hyperprogressive disease
Hyperprogressive disease (HPD) is defined as an atypical acceleration of tumor growth kinetics leading to premature death (Fig. 1b), which may occur following immunotherapy treatment [54,55,56]. Between 4% [57] and 29% [58] of patients with solid tumors will develop an augmented progression profile leading to a doubling of tumor burden and/or a twofold increase in tumor growth rate during ICI. There is currently no consensus on the precise criteria by which to define HPD, since previous studies used different methods of tumor burden assessment (for example, the sum of the largest diameters, tumor volume measurement) and different thresholds of tumor growth kinetics. In other cases, also a time to treatment failure under 2 months has been used to define HPD [57, 58]. Due to its recent emergence as a clinical phenomenon, HPD may be underdiagnosed. Therefore, the underlying mechanism of development represents an area of active investigation [59]. Some risk factors for HPD have been, however, described, and they include higher age [56] and the presence of MDM2/4 (murine double minute 2/4) family amplification or EGFR ( epidermal growth factor receptor) aberrations [57].
Durable response
Depending on tumor and ICI type, 10 to 25% of patients with metastatic cancer will achieve a durable tumor response (Fig. 1d) that can be maintained several years even after stopping the treatment [60, 61]. A recent pooled analysis of phase III trials found that the proportion of patients who experienced a durable response was 2.3 times higher in those treated with an ICI compared with those treated by standard chemo/targeted therapies in the control arms (25% vs 11%) [62]. There is currently no standardized definition of durable response, since the criteria of durable response differ across studies.
Clinical indications
The use of [18F]FDG PET/CT in the context of immunotherapeutic regimens should be considered at various time points related to treatment, based on clinical requirements. In particular:
-
Before start of treatment
At baseline, [18F]FDG PET/CT should be considered mandatory for tumor assessment, for [18F]FDG-avid tumors, particularly in case of first-line immunotherapeutic regimens, since it provides a basis for tumor monitoring or a confirmation of disease progression/recurrence.
Defining target lesions, the most intense sites of [18F]FDG uptake (e.g., SUVmax, SUVpeak) and computation of volumetric parameters (e.g., MTV) are recommended at baseline, to act as a basis for monitoring disease response at a later time.
-
During the course of treatment
[18F]FDG PET/CT is recommended at interim, commonly 8-12 weeks (i.e., 3-4 cycles) after treatment start, in particular to complement the information obtained from morphological imaging with CT and to resolve discordant findings.
The PET/CT scan can be also performed earlier or later during the course of treatment in case of clinical deterioration and/or suspicious progression on contrast-enhanced CT.
-
Before immunotherapy discontinuation
In patients receiving maintenance therapy or undergoing long-term treatment with ICIs, [18F]FDG PET/CT may be obtained to assess metabolic response, particularly in partial responders or stable disease on CT [63].
In patients requiring a temporary interruption of immunotherapy, [18F]FDG PET/CT restaging is recommended before restarting the treatment to reestablish a new baseline for subsequent response assessment.
Response criteria
A wide range of response criteria to ICI (Table 1) have been proposed, typically relying on ceCT scans. Although these criteria account for pseudoprogression, they do not encompass the complexity of managing patients with ICIs due to several new patterns of response and progression described below. In addition, a wide range of treatment combinations is currently being explored. These extend from systemic ICIs monotherapy to combinations with chemotherapy or targeted therapies in cases with relevant tumor mutations (e.g., BRAF/MEK in melanoma and EGFR in non-small cell lung cancer, NSCLC). Other treatment variations include localized delivery of ICIs through intraarterial perfusion, local treatments with intratumoral immunotherapy [64] and radiotherapy–immunotherapy combinations [65, 66].
At a clinical trial level, it has been demonstrated that reclassifying pseudoprogressive patients as treatment-sensitive avoids a potential bias that would otherwise favor chemotherapeutic alternatives based on progression-free survival (PFS), when, in fact, such patients may subsequently achieve prolonged survival. This phenomenon led to the refinement of standard response evaluation guidelines in the form of irRC [22], iRECIST [21] and irRECIST [69] for solid tumors. To prevent patients with PPD from prematurely terminating treatment, these guidelines propose a “wait-and-see” strategy (reevaluation using a follow-up scan 4–8 weeks later), when tumor burden appears to increase on imaging without significant clinical deterioration.
The interpretation of [18F]FDG PET/CT in patients treated with immunotherapy has to take into account this PPD phenomenon. Hence, several metabolic response criteria have been proposed as an alternative to PERCIST criteria [68]. These criteria are summarized below. Currently, there are insufficient data to decide which of these different approaches to categorize response is preferable. Furthermore, the impact on long-term patient outcomes has not been prospectively validated in randomized clinical trials.
In advanced melanoma treated with ICIs, PET/CT Criteria for Early Prediction of Response to Immune checkpoint inhibitor Therapy (PECRIT), which combines morphological (RECIST) and metabolic criteria, categorized 20 patients on the presence or absence of clinical benefit with a 100% sensitivity and a 93% specificity [24].
In an attempt to tackle the pitfalls and limitations of [18F]FDG PET imaging—foremost the phenomenon of pseudoprogression—in the assessment of immunotherapy response in melanoma, another set of modified response criteria has been developed, the PET Response Evaluation Criteria for IMmunoTherapy (PERCIMT). The cornerstone of PERCIMT is the finding that the changes in the absolute number of [18F]FDG-avid lesions are more predictive of clinical outcome than their respective standardized uptake value (SUV) changes during therapy with ICIs [26]. More specifically, according to these criteria, neither a mere increase in SUV of the target/index lesion(s) nor the development of one new hypermetabolic lesion in follow-up [18F]FDG PET/CT scan mean disease progression per se, as suggested by the conventional PERCIST/EORTC criteria [67, 68]. Instead, the application of a threshold of four newly emerged, [18F]FDG-avid lesions—with a decreasing cutoff of lesion number as the functional diameter of the lesions increases—can more correctly classify patients with progressive disease. More specifically, PMD is determined as the appearance of either four or more new lesions < 1 cm in functional diameter, or three or more new lesions > 1.0 cm in functional diameter, or two or more new lesions > 1.5 cm in functional diameter [26]. Otherwise, the patient can be classified as in PPD (Table 1).
PERCIMT criteria were developed in a metastatic melanoma cohort of 41 patients treated with ipilimumab, undergoing [18F]FDG PET/CT imaging before and after the end of ipilimumab treatment, and using the patients’ clinical response as reference [26]. They have been further validated in melanoma cohorts under different immunotherapeutic regimens and combinations both early during treatment (after two cycles of ICIs) [70, 71] and at the end of it (four cycles of ipilimumab and ipilimumab/vemurafenib treatment) [72, 73] yielding a satisfactory preliminary performance in patient stratification, predominantly in comparison with EORTC. However, further evaluation of the newly proposed criteria in larger patient cohorts is warranted, preferably in correlation with other metabolic or volumetric [18F]FDG parameters, such as MTV and total lesion glycolysis (TLG), as well as clinical and laboratory data.
Derived from PERCIST, imPERCIST differs in that the appearance of new lesions is not sufficient to classify a patient as having progressive disease. In these criteria, the peak standardized uptake value normalized for lean body mass (SULpeak) of up to five lesions on the baseline and follow-up scan is summed for each scan (maximum of 2 per organ). Target lesions on follow-up scans are the most intense lesions and are not necessarily the same as target lesions at baseline. PMD is defined as an increase of the sum of SULpeak by at least 30%. The appearance of new lesions is not sufficient to define PMD, but new lesions are included in the sum of SULpeak only if they show higher uptake than preexisting target lesions or if fewer than five target lesions were detected on the baseline scan. The prognostic value of imPERCIST criteria slightly outperformed those of standard PERCIST criteria [28].
Finally, a few recent studies have chosen to adapt the PERCIST criteria to the “wait-and-see” approach initially proposed by the iRECIST guidelines, leading to the so-called iPERCIST criteria [29, 48, 74]. Patients with new lesions or increase of more than 30% of the sum of SULpeak or of the SULpeak of the most intense lesions are classified as having unconfirmed progressive metabolic disease (uPMD). Then, if patients are clinically stable, reevaluation 4 to 8 weeks later is needed to establish confirmed progressive metabolic disease (cPMD). These studies have found that, for patients with metastatic lung cancer having uPMD on the first interim PET, the subsequent confirmatory PET reclassifies around one-third of these early-progressing patients as patients with atypical response patterns (PPD or dissociated response) who will in fact benefit from continuation of ICIs. Thus, it underlines the risk of falsely concluding to treatment failure after a first uPMD, a risk that is higher with [18F]FDG PET/CT than with ceCT due to its high sensitivity in detecting immune cell activation.
As general recommendation from this guideline/procedure standard, in case doubts exist between progression or pseudoprogression, especially on the first post-treatment evaluation, a confirmatory follow-up [18F]FDG PET/CT study 4–8 weeks later in the setting of clinical stability should be performed (Figs. 2 and 3). This consensus stems from the fact that there is no robust and externally validated tool to differentiate true progression from pseudoprogression based upon a single imaging assessment. Hence, treatment should be continued in clinically stable patients, absent excessive toxicity, to avoid discontinuation of ICIs in patients who may exhibit clinical benefit and objective response at a later time point.
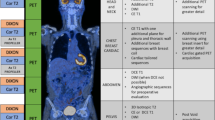
Illustration of target selection and [18F]FDG PET/CT response evaluation in a patient with multiple lesions and a dissociated response. Serial [18F]FDG MIP in a 73-year-old woman affected by a metastatic melanoma of the anal canal. (a) PET baseline before introduction of immunotherapy and (b) after six courses of nivolumab, showing a dissociated response with (i) progression of the main liver lesion, (ii) good response in the largest nodal lesion (right pulmonary hilum) and (iii) appearance of new lesions (liver, thoracic node, vertebral bone lesion; green arrows). This appearance of new lesions classifies the patient with progressive metabolic disease (PMD) according to the PERCIST criteria. As opposed to PERCIST, in imPERCIST5 (immunotherapy-modified PERCIST, five-lesion analysis), the appearance of new lesions alone does not result in PMD: PMD is defined only by an increase of the sum of SULpeaks by 30%, and new lesions are included in the sum of SULpeak if they show higher uptake than existing target lesions or if fewer than five target lesions were detected on the baseline scan. In the present case, a mediastinal node and a new bone lesion (green arrows) are selected, together with the three preexisting lesions (panel b). The patient is also classified as PMD according to imPERCIST. Follow-up scans at 1 and 4 months show clear progression (c). Summary table of target lesions, SULpeak values and their variation according to imPERCIST5 criteria are shown in panel (d)
PERCIST, iPERCIST, imPERCIST and PERCIMT evaluation in a patient with pseudoprogression at early evaluation. Serial [18F]FDG MIP in a 66-year-old woman affected by a metastatic cutaneous melanoma. (a, b) MIP and transaxial slices at baseline before introduction of immunotherapy and after two courses of nivolumab (c-f), showing two new lung lesions (d, f; green arrows) as well as a progression in tracer uptake and RECIST measurements of the main lung metastasis (e; red arrows). This pattern classifies the patient with progressive metabolic disease (PMD) according to the PERCIST criteria, and uPMD based on iPERCIST criteria. imPERCIST including the two hottest lung lesions (e, f) also classifies the patient as PMD, due to an increase in the sum of SULpeak greater than 30%. According to PERCIMT, the patient is classified as SMD (appearance of two new lesions, the size of which is <1.5 cm). Follow-up scan shows complete disappearance of lung lesions, classifying the patient as CMR and retrospectively the early evaluation as pseudoprogression. Also noteworthy is the appearance of a diffuse colic uptake suggestive of colitis, confirmed also by wall thickening that is usually detected on CT images (g) and serves for the differential diagnosis between metformin-induced colon uptake from immune-related colitis [75]. This patient had a 23-month progression-free survival (PFS) and experienced a recurrence in the peritoneum and right adrenal gland with no active disease at the thoracic level
Assessing immune organs and irAEs
In addition to the [18F]FDG PET/CT response criteria cited above, that were created to meet the challenges raised by immunotherapy, several groups have reported baseline prognostic factors of response such as the MTV [76] and uptake in immune organs [77].
The first sign of immune activity to be evaluated is spleen enlargement and/or increased uptake leading to an equalization or an inversion of the spleen-to-liver ratio (SLR) [39, 78]. Some groups also proposed other signs such as the bone marrow-to-liver ratio (BLR) [79] and uptake in the ileocecal valve [80]. While the attention of the PET community has been mainly focused on the capability of SLR to predict immune activation [34, 78] (an increased spleen uptake being considered to reflect “unleashed” T lymphocytes with an expected better outcome), several studies (Table 2) showed that an increase in SLR on baseline or follow-up [18F]FDG PET was an unfavorable finding, likely related to inflammation and tumor burden.
Strong reproducibility was reported for spleen and bone marrow measurements [81].
In addition to signs of immune activation and conventional or ICI-adapted [18F]FDG PET response criteria, the third cornerstone is evaluating the occurrence of irAEs. While several studies have shown that patients experiencing irAEs may have better survival [86, 87], studies on PET-detected irAEs are scarce [88, 89]. Lang et al. observed in a melanoma cohort under ipilimumab a significant correlation between PET signs of colitis and clinically significant diarrhea, although neither PET-colitis nor diarrhea was significantly correlated with response to therapy [90]. Recently, Wong et al. showed that [18F]FDG PET/CT could often detect relevant irAEs which may precede clinical diagnosis in melanoma patients receiving a combination of two ICIs (Table 3) [39, 91].
[18F]PET/CT protocols
[18F]FDG PET/CT Acquisition
[18F]FDG PET/CT procedure should be performed as described in the EANM guideline [98] and SNMMI procedure standards for tumor imaging [99]. The RSNA QIBA FDG/CT guidance is largely concordant and also acceptable [100].
Briefly, fasting is recommended for at least 6 h, and the acquisition should be performed following an interval of 60 min from tracer injection (acceptable range of 55 – 75 min) [101] by using as default mode a torso imaging (from the skull base to the mid-thighs). Including the skull base at least for therapy assessment examinations is recommended, so that immune-related hypophysitis can be detected. An extended whole-body imaging, covering from the vertex through the feet, can be indicated in case of neoplasia with clinical suspicion of more extensive metastatic disease (e.g., NSCLC, melanoma, Merkel cell tumor, etc.). [18F]FDG PET/CT with diagnostic CT and/or contrast-enhancement can be used following the acquisition parameters determined according to specific radiological society guidelines [98, 102].
In case of repeated [18F]FDG PET/CT, especially in case of therapy response assessment, the scan should be performed with identical acquisition and reconstruction parameters, by maintaining stringent uptake intervals from tracer administration to image acquisition. In case of facilities with multiple tomographs, the repeated scan should be performed on the same machine. In any case, all scanners should comply with international harmonizing standards, such as the EANM/EARL program [98, 103].
Data extraction and analysis
Quantitative PET/CT can be used as a diagnostic or prognostic tool (i.e., single measurement) or for therapy monitoring (i.e., longitudinal studies). Metrics include standardized uptake value (peak or max) computed either using bodyweight (SUV) or lean body mass (SUL) as normalizing measure for distribution volume, metabolic tumor volume (MTV) and total lesion glycolysis (TLG), defined as MTV × SUVmean. MTV is the volume inside a user- or algorithm-defined volume of interest (VOI) used to circumscribe the metabolically active tumor. Several techniques have been proposed to determine the limits of the VOI, threshold-based or algorithm-based [104], while TLG incorporates both [18F]FDG uptake and size of the tumor, also known as whole metabolic burden of the tumor [105]. The same method should be used to evaluate all scans—baseline and subsequent scans—in a patient as variability in MTV determinations by varying methods is well known.
Use of quantitative [18F]FDG PET/CT parameters for therapy monitoring purposes requires that these parameters are comparable among patients, regardless of the PET/CT system used. Therapy response criteria using SUVmax and, to a lesser extent, SUVpeak are affected by reconstruction inconsistencies between baseline and post-treatment scans [106, 107], which may arise when scanning patients in centers running several PET systems or as a result of patients’ mobility requiring scans at a different facility. Delineation of MTVs may be affected by the same errors as for SUVs, with variability in tumor delineation methodology being one of the major sources of variability [108]. It is therefore recommended to comply with harmonizing standards such as the EANM/EARL program, one of the international harmonization programs aiming at using [18F]FDG PET/CT as a quantitative imaging biomarker [98, 103].
Documentation and reporting
General recommendations for [18F]FDG PET/CT documentation and reporting are available in the EANM guideline for tumor imaging [98] and SNMMI procedure standards [99]. Special considerations when dealing with ICIs are highlighted in the following.
-
Clinical information
The clinical history of the patient should be briefly summarized, including relevant diagnostic tests and prior imaging findings. The type and number of cycles of ICIs must be specified, including the date of the most recent administration. Concomitant drug and treatments potentially impacting [18F]FDG uptake should be listed. This particularly includes metformin to avoid misinterpreting increased colonic uptake as evidence of immunotherapy-related colitis [109]
-
Technical Details
Details of the administered activity, uptake interval, scanner used and blood glucose level should be recorded to allow these to be preferably emulated or, at least, compared for follow-up scans.
-
Description of the findings
Imaging findings should be described in a logical manner, preferably following relevance over clinical indication. Findings may be grouped by significance, TNM staging or described by body region. For important [18F]FDG findings, the location, extent and intensity of [18F]FDG uptake should be described, as well as the relevant morphological findings on CT.
Target lesions should be identified following the indications of the chosen metabolic response criteria (Table 1) and reported, including longest diameter on axial view and SUV/SUL parameters (i.e., max, peak). In case of tumor response assessment, the pattern of metabolic response criteria used for the computation must be specified and documented.
Comparison of current findings with prior scans and other comparative imaging, when available, must be performed and reported.
The appearance, extent, severity and variation over time of the irAEs and other signs of immune activation (Tables 2, 3, and 4) must be described, preferably in a separate section of the report [34, 78, 110, 111].
-
Impression/conclusions
According to the clinical indication and timing, the conclusive remarks must be reported. Tumor extent or staging, treatment metabolic response and most relevant irAEs must be clearly stated. The complex nature of immune response seen with [18F]FDG PET/CT should be taken into consideration when performing conclusive remarks, with serial measurements and clinical response to be correlated. Ideally, there should be a focus on findings of clinical significance, particularly with respect to any additional diagnostic studies or subsequent scanning that might be required to clarify or confirm, for instance, the impression of disease progression (Table 4). Decisions based on the scan findings alone may be less accurate.
-
Direct communication
In addition to inclusion in the report conclusion, any significant abnormalities and severe irAEs [37, 39, 78] should be verbally communicated to the appropriate healthcare provider, in order to optimize patient management and avoid treatment delays that otherwise might result in significant morbidity or mortality. Reporting of abnormalities requiring urgent attention should be consistent with the policy of the interpreting physician’s local organization and the pathway for verbal communication.
-
Additional remarks
Taking into consideration that therapy assessment of cancer patients receiving ICIs (i) is often made in the context of busy PET centers and therefore should use user-friendly and reliable PET metrics, (ii) a consensus on treating patients beyond disease progression has been reached in certain tumor types and (iii) [18F]FDG PET/CT for assessment of immunotherapy is a dynamic field of research, these guidelines will provide recommendations on the use of the most clinically validated criteria, with metrics to be gathered for future pooled multicentric research. Participation of PET readers in tumor boards is crucial for patient care. Furthermore, [18F]FDG PET should be included in prospective randomized clinical trials in order to determine whether [18F]FDG PET-based response assessment can predict the effectiveness of a specific drug regimen more accurately than response assessment by RECIST. It should be recognized that the use of PET may alter the rate of detecting irAEs compared to that previously identified in routine clinical care and may impact both subsequent treatments that might be administered for these complications and the longer-term consequences.
The implementation of artificial intelligence (AI) for image analysis with the development of dedicated algorithms for PET image segmentation as well as AI-based evaluation of follow-up studies will facilitate therapy monitoring with [18F]FDG PET–CT in future [112,111,114]. Additionally, the translation of dedicated tracers into clinical routine, like labeled T cells, or labeled PD-1 or PD-L1 antibodies will provide complementary information to [18F]FDG and improve prediction to immunotherapy response [115].
Checklist
Table 4 highlights the checklist of requirements for patients with solid tumors treated with ICI.
Liability statement
This guideline summarizes the views of the EANM Oncology and Theranostics Committee, the EANM Inflammation and Infection Committee, the SNMMI and the ANZSNM. It reflects recommendations for which the EANM cannot be held responsible. The recommendations should be taken into context of good practice of nuclear medicine and do not substitute for national and international legal or regulatory provisions.
Abbreviations
- AI:
-
Artificial Intelligence
- ANZSNM:
-
Australian and New Zealand Society of Nuclear Medicine
- BLR:
-
Bone marrow-to-liver ratio
- BRAF:
-
B Rapidly Accelerated Fibrosarcoma
- CMR:
-
Complete metabolic response
- cPMD:
-
Confirmed progressive metabolic disease
- CR:
-
Complete response (anatomical)
- ceCT:
-
Contrast-enhanced CT
- CT:
-
Computed tomography
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein 4
- DR:
-
Dissociated response
- EANM:
-
European Association of Nuclear Medicine
- EARL:
-
EANM Research Ltd
- EGFR:
-
Epidermal growth factor receptor
- EORTC:
-
European Organization for Research and Treatment of Cancer
- FD:
-
Fractal dimension
- FDG:
-
2-[18F]fluoro-2-deoxy-D-glucose
- HPD:
-
Hyperprogressive disease
- ICIs:
-
Immune checkpoint inhibitors
- IDDM:
-
Insulin-Dependent Diabetes Mellitus
- imPERCIST:
-
Immunotherapy-modified PERCIST
- irAEs:
-
Immune-related adverse events
- iRECIST:
-
Modified RECIST 1.1 for immune-based therapeutics
- irRECIST:
-
Immune-related Response Evaluation Criteria in Solid Tumors
- MDM 2/4:
-
Murine double minute 2/4
- MEK:
-
Mitogen-activated protein kinase
- MIP:
-
Maximal intensity projection
- MTV:
-
Metabolic tumor volume
- NSCLC:
-
Non-small cell lung cancer
- PECRIT:
-
PET/CT Criteria for Early Prediction of Response to Immune checkpoint inhibitor Therapy
- PERCIMT:
-
PET Response Evaluation Criteria for IMmunoTherapy
- PERCIST:
-
Positron Emission Tomography Response Criteria In Solid Tumors
- PD:
-
Progressive disease
- PD-1:
-
Programmed cell death-associated protein 1
- PD-L1:
-
Programmed death ligand 1
- PET/CT:
-
Positron emission tomography/computed tomography
- PFS:
-
Progression-free survival
- PMD:
-
Progressive metabolic disease
- PMR:
-
Partial metabolic response
- PPD:
-
Pseudoprogressive disease
- PR:
-
Partial response
- QIBA:
-
Quantitative Imaging Biomarkers Alliance
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- RSNA:
-
Radiological Society of North America
- SD:
-
Stable disease
- SLR:
-
Spleen-to-liver ratio
- SMD:
-
Stable metabolic disease
- SNMMI:
-
Society of Nuclear Medicine and Molecular Imaging
- SUL:
-
Standardized uptake value normalized by lean body mass
- SUV:
-
Standardized uptake value
- TLG:
-
Total lesion glycolysis
- TMTV:
-
Total metabolic tumor volume
- TNM:
-
Tumor (T), nodes (N), and metastases (M)
- uPMD:
-
Unconfirmed progressive metabolic disease
- VOI:
-
Volume of interest
References
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. https://doi.org/10.1056/NEJMoa1003466.
Rossi S, Toschi L, Castello A, Grizzi F, Mansi L, Lopci E. Clinical characteristics of patient selection and imaging predictors of outcome in solid tumors treated with checkpoint-inhibitors. Eur J Nucl Med Mol Imaging. 2017;44(13):2310–25. https://doi.org/10.1007/s00259-017-3802-5.
Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521–32. https://doi.org/10.1056/NEJMoa1503093.
Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–17. https://doi.org/10.1056/NEJMoa1414428.
Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for first-line treatment of PD-L1–selected patients with NSCLC. N Engl J Med. 2020;383:1328–39. https://doi.org/10.1056/NEJMoa1917346.
Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374–85. https://doi.org/10.1016/s1470-2045(16)30364-3.
Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39:98–106. https://doi.org/10.1097/COC.0000000000000239.
Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480–92. https://doi.org/10.1016/S1470-2045(18)30700-9.
George S, Rini BI, Hammers HJ. Emerging role of combination immunotherapy in the first-line treatment of advanced renal cell carcinoma: a review. JAMA Oncol. 2019;5:411–21. https://doi.org/10.1001/jamaoncol.2018.4604.
Stewart RA, Pilie PG, Yap TA. Development of PARP and immune-checkpoint inhibitor combinations. Cancer Res. 2018;78:6717–25. https://doi.org/10.1158/0008-5472.CAN-18-2652.
Galluzzi L, Humeau J, Buqué A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17:725–41. https://doi.org/10.1038/s41571-020-0413-z.
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med. 2017;377:1919–29. https://doi.org/10.1056/NEJMoa1709937.
Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med. 2017;377:1824–35. https://doi.org/10.1056/NEJMoa1709030.
Keung EZ, Ukponmwan EU, Cogdill AP, Wargo JA. The Rationale and emerging use of neoadjuvant immune checkpoint blockade for solid malignancies. Ann Surg Oncol. 2018;25:1814–27. https://doi.org/10.1245/s10434-018-6379-8.
Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson VG, Dalle S, et al. Adjuvant Pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22:643–54. https://doi.org/10.1016/S1470-2045(21)00065-6.
Haas L, Wiesner T, Obenauf AC. A new era of proactive melanoma therapy: hit hard, hit early. Br J Dermatol. 2018;178:817–20. https://doi.org/10.1111/bjd.16347.
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. https://doi.org/10.1016/j.immuni.2013.07.012.
Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. https://doi.org/10.1158/1078-0432.CCR-09-1624.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res. 2013;19:3936–43. https://doi.org/10.1158/1078-0432.Ccr-13-0895.
Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–52. https://doi.org/10.1016/s1470-2045(17)30074-8.
Hodi FS, Ballinger M, Lyons B, Soria JC, Nishino M, Tabernero J, et al. Immune-modified response evaluation criteria in solid tumors (imRECIST): refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol. 2018;36:850–8. https://doi.org/10.1200/JCO.2017.75.1644.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. https://doi.org/10.1126/science.1160809.
Cho SY, Lipson EJ, Im HJ, Rowe SP, Gonzalez EM, Blackford A, et al. Prediction of response to immune checkpoint inhibitor therapy using early-time-point (18)F-FDG PET/CT imaging in patients with advanced melanoma. J Nucl Med. 2017;58:1421–8. https://doi.org/10.2967/jnumed.116.188839.
Sachpekidis C, Larribere L, Pan L, Haberkorn U, Dimitrakopoulou-Strauss A, Hassel JC. Predictive value of early 18F-FDG PET/CT studies for treatment response evaluation to ipilimumab in metastatic melanoma: preliminary results of an ongoing study. Eur J Nucl Med Mol Imaging. 2015;42:386–96. https://doi.org/10.1007/s00259-014-2944-y.
Anwar H, Sachpekidis C, Winkler J, Kopp-Schneider A, Haberkorn U, Hassel JC, et al. Absolute number of new lesions on (18)F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur J Nucl Med Mol Imaging. 2018;45:376–83. https://doi.org/10.1007/s00259-017-3870-6.
Cheson BD, Ansell S, Schwartz L, Gordon LI, Advani R, Jacene HA, et al. Refinement of the Lugano classification lymphoma response criteria in the era of immunomodulatory therapy. Blood. 2016;128:2489–96. https://doi.org/10.1182/blood-2016-05-718528.
Ito K, Teng R, Schoder H, Humm JL, Ni A, Michaud L, et al. (18)F-FDG PET/CT for monitoring of ipilimumab therapy in patients with metastatic melanoma. J Nucl Med. 2019;60:335–41. https://doi.org/10.2967/jnumed.118.213652.
Goldfarb L, Duchemann B, Chouahnia K, Zelek L, Soussan M. Monitoring anti-PD-1-based immunotherapy in non-small cell lung cancer with FDG PET: introduction of iPERCIST. EJNMMI Res. 2019;9:8. https://doi.org/10.1186/s13550-019-0473-1.
Grizzi F, Castello A, Lopci E. Is it time to change our vision of tumor metabolism prior to immunotherapy? Eur J Nucl Med Mol Imaging. 2018;45(6):1072–5. https://doi.org/10.1007/s00259-018-3988-1.
Castello A, Rossi S, Toschi L, Lopci E. Comparison of metabolic and morphological response criteria for early prediction of response and survival in NSCLC patients treated with anti-PD-1/PD-L1. Front Oncol. 2020;31(10):1090. https://doi.org/10.3389/fonc.2020.01090.
Castello A, Toschi L, Rossi S, Mazziotti E, Lopci E. The immune-metabolic-prognostic index and clinical outcomes in patients with non-small cell lung carcinoma under checkpoint inhibitors. J Cancer Res Clin Oncol. 2020;146(5):1235–43. https://doi.org/10.1007/s00432-020-03150-9.
Castello A, Rossi S, Mazziotti E, Toschi L, Lopci E. Hyperprogressive disease in patients with non-small cell lung cancer treated with checkpoint inhibitors: the role of 18F-FDG PET/CT. J Nucl Med. 2020;61(6):821–6. https://doi.org/10.2967/jnumed.119.237768.
Wong ANM, McArthur GA, Hofman MS, Hicks RJ. The advantages and challenges of using FDG PET/CT for response assessment in melanoma in the era of targeted agents and immunotherapy. Eur J Nucl Med Mol Imaging. 2017;44:67–77. https://doi.org/10.1007/s00259-017-3691-7.
Palmer CS, Ostrowski M, Balderson B, Christian N, Crowe SM. Glucose metabolism regulates T cell activation, differentiation, and functions. Front Immunol. 2015;6:1. https://doi.org/10.3389/fimmu.2015.00001.
Ayati N, Sadeghi R, Kiamanesh Z, Lee ST, Zakavi SR, Scott AM. The value of 18F-FDG PET/CT for predicting or monitoring immunotherapy response in patients with metastatic melanoma: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2020. https://doi.org/10.1007/s00259-020-04967-9.
Iravani A, Osman MM, Weppler AM, Wallace R, Galligan A, Lasocki A, et al. FDG PET/CT for tumoral and systemic immune response monitoring of advanced melanoma during first-line combination ipilimumab and nivolumab treatment. Eur J Nucl Med Mol Imaging. 2020;47:2776–86. https://doi.org/10.1007/s00259-020-04815-w.
Ito K, Schoder H, Teng R, Humm JL, Ni A, Wolchok JD, et al. Prognostic value of baseline metabolic tumor volume measured on (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in melanoma patients treated with ipilimumab therapy. Eur J Nucl Med Mol Imaging. 2019;46:930–9. https://doi.org/10.1007/s00259-018-4211-0.
Wong A, Callahan J, Keyaerts M, Neyns B, Mangana J, Aberle S, et al. (18)F-FDG PET/CT based spleen to liver ratio associates with clinical outcome to ipilimumab in patients with metastatic melanoma. Cancer Imaging : the official publication of the International Cancer Imaging Society. 2020;20:36. https://doi.org/10.1186/s40644-020-00313-2.
Borcoman E, Kanjanapan Y, Champiat S, Kato S, Servois V, Kurzrock R, et al. Novel patterns of response under immunotherapy. Ann Oncol. 2019;30:385–96. https://doi.org/10.1093/annonc/mdz003.
Queirolo P, Spagnolo F. Atypical responses in patients with advanced melanoma, lung cancer, renal-cell carcinoma and other solid tumors treated with anti-PD-1 drugs: a systematic review. Cancer Treat Rev. 2017;59:71–8. https://doi.org/10.1016/j.ctrv.2017.07.002.
Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with Pembrolizumab. J Clin Oncol. 2016;34:1510–7. https://doi.org/10.1200/JCO.2015.64.0391.
Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol. 2015;33:3541–3. https://doi.org/10.1200/JCO.2015.61.6870.
Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–64. https://doi.org/10.1016/S1470-2045(09)70334-1.
Leibman BD, Dillioglugil O, Abbas F, Tanli S, Kattan MW, Scardino PT. Impact of a clinical pathway for radical retropubic prostatectomy. Urology. 1998;52:94–9. https://doi.org/10.1016/s0090-4295(98)00130-7.
Martin-Romano P, Castanon E, Ammari S, Champiat S, Hollebecque A, Postel-Vinay S, et al. Evidence of pseudoprogression in patients treated with PD1/PDL1 antibodies across tumor types. Cancer Med. 2020;9:2643–52. https://doi.org/10.1002/cam4.2797.
Bernard-Tessier A, Baldini C, Castanon E, Martin P, Champiat S, Hollebecque A, et al. Patterns of progression in patients treated for immuno-oncology antibodies combination. Cancer Immunol Immunother. 2021;70:221–32. https://doi.org/10.1007/s00262-020-02647-z.
Humbert O, Cadour N, Paquet M, Schiappa R, Poudenx M, Chardin D, et al. (18)FDG PET/CT in the early assessment of non-small cell lung cancer response to immunotherapy: frequency and clinical significance of atypical evolutive patterns. Eur J Nucl Med Mol Imaging. 2020;47:1158–67. https://doi.org/10.1007/s00259-019-04573-4.
Gerwing M, Herrmann K, Helfen A, Schliemann C, Berdel WE, Eisenblatter M, et al. The beginning of the end for conventional RECIST - novel therapies require novel imaging approaches. Nat Rev Clin Oncol. 2019;16:442–58. https://doi.org/10.1038/s41571-019-0169-5.
Humbert O, Chardin D. Dissociated response in metastatic cancer: an atypical pattern brought into the spotlight with immunotherapy. Front Oncol. 2020;10:566297. https://doi.org/10.3389/fonc.2020.566297.
Tazdait M, Mezquita L, Lahmar J, Ferrara R, Bidault F, Ammari S, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer. 2018;88:38–47. https://doi.org/10.1016/j.ejca.2017.10.017.
Tozuka T, Kitazono S, Sakamoto H, Yoshida H, Amino Y, Uematsu S, et al. Dissociated responses at initial computed tomography evaluation is a good prognostic factor in non-small cell lung cancer patients treated with anti-programmed cell death-1/ligand 1 inhibitors. BMC Cancer. 2020;20:207. https://doi.org/10.1186/s12885-020-6704-z.
Klemen ND, Wang M, Feingold PL, Cooper K, Pavri SN, Han D, et al. Patterns of failure after immunotherapy with checkpoint inhibitors predict durable progression-free survival after local therapy for metastatic melanoma. J Immunother Cancer. 2019;7:196. https://doi.org/10.1186/s40425-019-0672-3.
Kas B, Talbot H, Ferrara R, Richard C, Lamarque JP, Pitre-Champagnat S, et al. Clarification of definitions of hyperprogressive disease during immunotherapy for non-small cell lung cancer. JAMA Oncol. 2020;6:1039–46. https://doi.org/10.1001/jamaoncol.2020.1634.
Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4:1543–52. https://doi.org/10.1001/jamaoncol.2018.3676.
Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by Anti-PD-1/PD-L1. Clin Cancer Res. 2017;23:1920–8. https://doi.org/10.1158/1078-0432.CCR-16-1741.
Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23:4242–50. https://doi.org/10.1158/1078-0432.CCR-16-3133.
Saada-Bouzid E, Defaucheux C, Karabajakian A, Coloma VP, Servois V, Paoletti X, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2017;28:1605–11. https://doi.org/10.1093/annonc/mdx178.
Seban RD, Schwartz LH, Bonardel G, Dercle L. Diagnosis of hyperprogressive disease in patients treated with checkpoint inhibitors using (18)F-FDG PET/CT. J Nucl Med. 2020;61:1404–5. https://doi.org/10.2967/jnumed.120.242768.
Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–94. https://doi.org/10.1200/JCO.2014.56.2736.
Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III Trials (checkMate 017 and checkMate 057). J Clin Oncol. 2017;35:3924–33. https://doi.org/10.1200/JCO.2017.74.3062.
Pons-Tostivint E, Latouche A, Vaflard P, Ricci F, Loirat D, Hescot S, et al. Comparative analysis of durable responses on immune checkpoint inhibitors versus other systemic therapies: a pooled analysis of phase III trials. JCO Precis Oncol. 2019;3:1–10. https://doi.org/10.1200/PO.18.00114.
Tan AC, Emmett L, Lo S, Liu V, Kapoor R, Carlino MS, et al. FDG-PET response and outcome from anti-PD-1 therapy in metastatic melanoma. Ann Oncol. 2018;29:2115–20. https://doi.org/10.1093/annonc/mdy330.
Champiat S, Tselikas L, Farhane S, Raoult T, Texier M, Lanoy E, et al. Intratumoral immunotherapy: from trial design to clinical practice. Clin Cancer Res. 2021;27:665–79. https://doi.org/10.1158/1078-0432.CCR-20-0473.
Deutsch E, Chargari C, Galluzzi L, Kroemer G. Optimising efficacy and reducing toxicity of anticancer radioimmunotherapy. Lancet Oncol. 2019;20:e452–63. https://doi.org/10.1016/S1470-2045(19)30171-8.
Ratnayake G, Reinwald S, Shackleton M, Moore M, Voskoboynik M, Ruben J, et al. Stereotactic radiation therapy combined with immunotherapy against metastatic melanoma: long-term results of a phase 1 clinical trial. Int J Radiat Oncol Biol Phys. 2020;108:150–6. https://doi.org/10.1016/j.ijrobp.2020.05.022.
Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–82. https://doi.org/10.1016/s0959-8049(99)00229-4.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S-S150. https://doi.org/10.2967/jnumed.108.057307.
Le Lay J, Jarraya H, Lebellec L, Penel N. irRECIST and iRECIST: the devil is in the details. Ann Oncol. 2017;28:1676–8. https://doi.org/10.1093/annonc/mdx168.
Sachpekidis C, Kopp-Schneider A, Pan L, Papamichail D, Haberkorn U, Hassel JC, et al. Interim [(18)F]FDG PET/CT can predict response to anti-PD-1 treatment in metastatic melanoma. Eur J Nucl Med Mol Imaging. 2021;48:1932–43. https://doi.org/10.1007/s00259-020-05137-7.
Sachpekidis C, Anwar H, Winkler J, Kopp-Schneider A, Larribere L, Haberkorn U, et al. The role of interim (18)F-FDG PET/CT in prediction of response to ipilimumab treatment in metastatic melanoma. Eur J Nucl Med Mol Imaging. 2018;45:1289–96. https://doi.org/10.1007/s00259-018-3972-9.
Sachpekidis C, Kopp-Schneider A, Hakim-Meibodi L, Dimitrakopoulou-Strauss A, Hassel JC. 18F-FDG PET/CT longitudinal studies in patients with advanced metastatic melanoma for response evaluation of combination treatment with vemurafenib and ipilimumab. Melanoma Res. 2019;29:178–86. https://doi.org/10.1097/CMR.0000000000000541.
Annovazzi A, Vari S, Giannarelli D, Pasqualoni R, Sciuto R, Carpano S, et al. Comparison of 18F-FDG PET/CT criteria for the prediction of therapy response and clinical outcome in patients with metastatic melanoma treated with Ipilimumab and PD-1 inhibitors. Clin Nucl Med. 2020;45:187–94. https://doi.org/10.1097/RLU.0000000000002921.
Rossi G, Bauckneht M, Genova C, Rijavec E, Biello F, Mennella S, et al. Comparison between (18)F-FDG PET-based and CT-based criteria in non-small cell lung cancer patients treated with Nivolumab. J Nucl Med. 2020;61:990–8. https://doi.org/10.2967/jnumed.119.233056.
Lyall A, Vargas HA, Carvajal RD, Ulaner G. Ipilimumab-induced colitis on FDG PET/CT. Clin Nucl Med. 2012;37:629–30. https://doi.org/10.1097/RLU.0b013e318248549a.
Seban RD, Moya-Plana A, Antonios L, Yeh R, Marabelle A, Deutsch E, et al. Prognostic 18F-FDG PET biomarkers in metastatic mucosal and cutaneous melanoma treated with immune checkpoint inhibitors targeting PD-1 and CTLA-4. Eur J Nucl Med Mol Imaging. 2020. https://doi.org/10.1007/s00259-020-04757-3.
Ayati N, Sadeghi R, Kiamanesh Z, Lee ST, Zakavi SR, Scott AM. The value of (18)F-FDG PET/CT for predicting or monitoring immunotherapy response in patients with metastatic melanoma: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2020. https://doi.org/10.1007/s00259-020-04967-9.
Aide N, Hicks RJ, Le Tourneau C, Lheureux S, Fanti S, Lopci E. FDG PET/CT for assessing tumour response to immunotherapy: report on the EANM symposium on immune modulation and recent review of the literature. Eur J Nucl Med Mol Imaging. 2019;46:238–50. https://doi.org/10.1007/s00259-018-4171-4.
Seban RD, Nemer JS, Marabelle A, Yeh R, Deutsch E, Ammari S, et al. Prognostic and theranostic 18F-FDG PET biomarkers for anti-PD1 immunotherapy in metastatic melanoma: association with outcome and transcriptomics. Eur J Nucl Med Mol Imaging. 2019;46:2298–310. https://doi.org/10.1007/s00259-019-04411-7.
Dercle L, Seban RD, Lazarovici J, Schwartz LH, Houot R, Ammari S, et al. (18)F-FDG PET and CT scans detect new imaging patterns of response and progression in patients with Hodgkin Lymphoma treated by anti-programmed death 1 immune checkpoint inhibitor. J Nucl Med. 2018;59:15–24. https://doi.org/10.2967/jnumed.117.193011.
Prigent K, Lasnon C, Ezine E, Janson M, Coudrais N, Joly E, et al. Assessing immune organs on (18)F-FDG PET/CT imaging for therapy monitoring of immune checkpoint inhibitors: inter-observer variability, prognostic value and evolution during the treatment course of melanoma patients. Eur J Nucl Med Mol Imaging. 2021. https://doi.org/10.1007/s00259-020-05103-3.
Sachpekidis C, Larribere L, Kopp-Schneider A, Hassel JC, Dimitrakopoulou-Strauss A. Can benign lymphoid tissue changes in (18)F-FDG PET/CT predict response to immunotherapy in metastatic melanoma? Cancer Immunol Immunother. 2019;68:297–303. https://doi.org/10.1007/s00262-018-2279-9.
Seban RD, Nemer JS, Marabelle A, Yeh R, Deutsch E, Ammari S. Prognostic and theranostic 18F-FDG PET biomarkers for anti-PD1 immunotherapy in metastatic melanoma: association with outcome and transcriptomics. Eur J Nucl Med Mol Imaging. 2019;46(11):2298–310. https://doi.org/10.1007/s00259-019-04411-7.
Seban RD, Mezquita L, Berenbaum A, Dercle L, Botticella A, Le Pechoux C, et al. Baseline metabolic tumor burden on FDG PET/CT scans predicts outcome in advanced NSCLC patients treated with immune checkpoint inhibitors. Eur J Nucl Med Mol Imaging. 2020;47:1147–57. https://doi.org/10.1007/s00259-019-04615-x.
Seban RD, Moya-Plana A, Antonios L, Yeh R, Marabelle A, Deutsch E. Prognostic 18F-FDG PET biomarkers in metastatic mucosal and cutaneous melanoma treated with immune checkpoint inhibitors targeting PD-1 and CTLA-4. Eur J Nucl Med Mol Imaging. 2020;47(10):2301–12. https://doi.org/10.1007/s00259-020-04757-3.
Kotwal A, Kottschade L, Ryder M. PD-L1 Inhibitor-Induced Thyroiditis Is Associated with Better Overall Survival in Cancer Patients. Thyroid: official journal of the American Thyroid Association. 2020;30:177–84. https://doi.org/10.1089/thy.2019.0250.
Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol. 2018;4:374–8. https://doi.org/10.1001/jamaoncol.2017.2925.
Nobashi T, Baratto L, Reddy SA, Srinivas S, Toriihara A, Hatami N, et al. Predicting response to immunotherapy by evaluating tumors, lymphoid cell-rich organs, and immune-related adverse events using FDG-PET/CT. Clin Nucl Med. 2019;44:e272–9. https://doi.org/10.1097/rlu.0000000000002453.
Wachsmann JW, Ganti R, Peng F. Immune-mediated disease in ipilimumab immunotherapy of melanoma with FDG PET-CT. Acad Radiol. 2017;24:111–5. https://doi.org/10.1016/j.acra.2016.08.005.
Lang N, Dick J, Slynko A, Schulz C, Dimitrakopoulou-Strauss A, Sachpekidis C, et al. Clinical significance of signs of autoimmune colitis in (18)F-fluorodeoxyglucose positron emission tomography-computed tomography of 100 stage-IV melanoma patients. Immunotherapy. 2019;11:667–76. https://doi.org/10.2217/imt-2018-0146.
Wong A, Callahan J, Beresford J, Herschtal A, Fullerton S, Milne D. Spleen to liver ratio (SLR): novel PET imaging biomarker for prediction of overall survival after ipilimumab and anti-PD1 in patients with metastatic melanoma. J Clin Oncol. 2016;34:9523–33.
Baxi S, Yang A, Gennarelli RL, Khan N, Wang Z, Boyce L, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:k793. https://doi.org/10.1136/bmj.k793.
Cappelli LC, Gutierrez AK, Bingham CO 3rd, Shah AA. Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: a systematic review of the literature. Arthritis Care Res (Hoboken). 2017;69:1751–63. https://doi.org/10.1002/acr.23177.
Champiat S, Lambotte O, Barreau E, Belkhir R, Berdelou A, Carbonnel F, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016;27:559–74. https://doi.org/10.1093/annonc/mdv623.
Darnell EP, Mooradian MJ, Baruch EN, Yilmaz M, Reynolds KL. Immune-Related Adverse Events (irAEs): diagnosis, management, and clinical pearls. Curr Oncol Rep. 2020;22:39. https://doi.org/10.1007/s11912-020-0897-9.
Michot JM, Lazarovici J, Tieu A, Champiat S, Voisin AL, Ebbo M, et al. Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage? Eur J Cancer. 2019;122:72–90. https://doi.org/10.1016/j.ejca.2019.07.014.
Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chavez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. https://doi.org/10.1038/s41572-020-0160-6.
Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54. https://doi.org/10.1007/s00259-014-2961-x.
Delbeke D, Coleman RE, Guiberteau MJ, Brown ML, Royal HD, Siegel BA, et al. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med. 2006;47:885–95.
Kinahan PE, Perlman ES, Sunderland JJ, Subramaniam R, Wollenweber SD, Turkington TG, et al. The QIBA profile for FDG PET/CT as an imaging biomarker measuring response to cancer therapy. Radiology. 2020;294:647–57. https://doi.org/10.1148/radiol.2019191882.
Graham MM, Wahl RL, Hoffman JM, Yap JT, Sunderland JJ, Boellaard R, et al. Summary of the UPICT Protocol for 18F-FDG PET/CT imaging in oncology clinical trials. J Nucl Med. 2015;56:955–61. https://doi.org/10.2967/jnumed.115.158402.
Beckett KR, Moriarity AK, Langer JM. Safe use of contrast media: what the radiologist needs to know. Radiographics. 2015;35:1738–50. https://doi.org/10.1148/rg.2015150033.
Aide N, Lasnon C, Veit-Haibach P, Sera T, Sattler B, Boellaard R. EANM/EARL harmonization strategies in PET quantification: from daily practice to multicentre oncological studies. Eur J Nucl Med Mol Imaging. 2017;44:17–31. https://doi.org/10.1007/s00259-017-3740-2.
Im HJ, Bradshaw T, Solaiyappan M, Cho SY. Current methods to define metabolic tumor volume in positron emission tomography: which one is better? Nucl Med Mol Imaging. 2018;52:5–15. https://doi.org/10.1007/s13139-017-0493-6.
Im HJ, Pak K, Cheon GJ, Kang KW, Kim SJ, Kim IJ, et al. Prognostic value of volumetric parameters of (18)F-FDG PET in non-small-cell lung cancer: a meta-analysis. Eur J Nucl Med Mol Imaging. 2015;42:241–51. https://doi.org/10.1007/s00259-014-2903-7.
Lasnon C, Quak E, Le Roux PY, Robin P, Hofman MS, Bourhis D, et al. EORTC PET response criteria are more influenced by reconstruction inconsistencies than PERCIST but both benefit from the EARL harmonization program. EJNMMI Phys. 2017;4:17. https://doi.org/10.1186/s40658-017-0185-4.
Quak E, Le Roux PY, Lasnon C, Robin P, Hofman MS, Bourhis D, et al. Does PET SUV harmonization affect PERCIST response classification? J Nucl Med. 2016;57:1699–706. https://doi.org/10.2967/jnumed.115.171983.
Lasnon C, Enilorac B, Popotte H, Aide N. Impact of the EARL harmonization program on automatic delineation of metabolic active tumour volumes (MATVs). EJNMMI Res. 2017;7:30. https://doi.org/10.1186/s13550-017-0279-y.
Hamidizadeh R, Eftekhari A, Wiley EA, Wilson D, Alden T, Benard F. Metformin discontinuation prior to FDG PET/CT: a randomized controlled study to compare 24- and 48-hour bowel activity. Radiology. 2018;289:418–25. https://doi.org/10.1148/radiol.2018180078.
Hicks RJ, Iravani A, Sandhu S. (18)F-fluorodeoxyglucose positron emission tomography/computed tomography for assessing tumor response to immunotherapy in solid tumors: melanoma and beyond. PET Clin. 2020;15:11–22. https://doi.org/10.1016/j.cpet.2019.08.007.
Prigent K, Aide N. (18)F-Fludeoxyglucose PET/Computed Tomography for assessing tumor response to immunotherapy and detecting immune-related side effects: a checklist for the PET reader. PET Clin. 2020;15:1–10. https://doi.org/10.1016/j.cpet.2019.08.006.
Aide N, Lasnon C, Kesner A, Levin CS, Buvat I, Iagaru A, et al. New PET technologies - embracing progress and pushing the limits. Eur J Nucl Med Mol Imaging. 2021;48:2711–26. https://doi.org/10.1007/s00259-021-05390-4.
Jh O, Lim SJ, Wang H, Leal JP, Shu HG, Wahl RL, et al. Quantitation of cancer treatment response by 2-[(18)F]FDG PET/CT: multi-center assessment of measurement variability using AUTO-PERCIST. EJNMMI Res. 2021;11:15. https://doi.org/10.1186/s13550-021-00754-1.
Slart R. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur J Nucl Med Mol Imaging. 2018;45:1250–69. https://doi.org/10.1007/s00259-018-3973-8.
Iravani A, Hicks RJ. Imaging the cancer immune environment and its response to pharmacologic intervention, Part 2: the role of novel PET agents. J Nucl Med. 2020;61:1553–9. https://doi.org/10.2967/jnumed.120.248823.
Acknowledgements
The guideline was brought to the attention of the relevant EANM Committees and the National Societies of Nuclear Medicine. The comments and suggestions from the EANM Neuroimaging and Technologists Committees, the SNMMI Councils and Public Review and the British Society of Nuclear Medicine are highly appreciated and have been considered for this Guideline.
Funding
The support for the open-access publication of the current manuscript was provided by the IRCCS – Humanitas Research Hospital, Rozzano (MI), Italy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflicts of interest
E.L. reports receiving grants from AIRC (Associazione Italiana per la Ricerca sul Cancro) and from the Italian Ministry of Health and faculty remuneration from ESMIT (European School of Multimodality Imaging and Therapy) and MI&T congressi. R. J.H. is on the Scientific Advisory Board of Telix Pharmaceuticals with any honoraria donated to his institution, he is a stock holder in this company, and he is also an honorary Trustee of the International Cancer Imaging Society and honorary Board Member of Neuroendocrine Cancer Australia. W.A.W. has been on the advisory boards and receives compensation from Blue Earth Diagnostics. N.P. declares honoraria from Actinium Pharmaceuticals, AstraZeneca/MedImmune; Consulting or Advisory Role from Illumina, progenics; Speakers' Bureau from Actinium Pharmaceuticals; Research Funding from Bayer Health; Bristol-Myers Squibb; Clarity Pharmaceuticals; Imaginab; Janssen; Regeneron; Travel, Accommodations Expenses from Bayer. The other authors declare no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology—General
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lopci, E., Hicks, R.J., Dimitrakopoulou-Strauss, A. et al. Joint EANM/SNMMI/ANZSNM practice guidelines/procedure standards on recommended use of [18F]FDG PET/CT imaging during immunomodulatory treatments in patients with solid tumors version 1.0. Eur J Nucl Med Mol Imaging 49, 2323–2341 (2022). https://doi.org/10.1007/s00259-022-05780-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05780-2