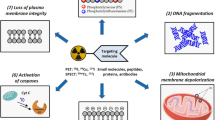
Abstract
Positron emission tomography (PET) imaging of apoptosis can noninvasively detect cell death in vivo and assist in monitoring tumor response to treatment in patients. While extensive efforts have been devoted to addressing this important need, no apoptosis PET imaging agents have yet been approved for clinical use. This study reports an improved 18F-labeled caspase-sensitive nanoaggregation tracer ([18F]-C-SNAT4) for PET imaging of tumor response to chemo- and immunotherapies in preclinical mouse models.
Methods
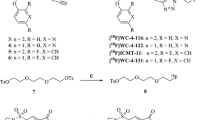
We rationally designed and synthesized a new PET tracer [18F]-C-SNAT4 to detect cell death both in vitro and in vivo. In vitro radiotracer uptake studies were performed on drug-sensitive and -resistant NSCLC cell lines (NCI-H460 and NCI-H1299, respectively) treated with cisplatin at different doses. In vivo therapy response monitoring by [18F]-C-SNAT4 PET imaging was evaluated with two treatment modalities—chemotherapy and immunotherapy in two tumor xenografts in mice. Radiotracer uptake in the tumors was validated ex vivo using γ-counting and cleaved caspase-3 immunofluorescence.
Results
This [18F]-C-SNAT4 PET tracer was facilely synthesized and displayed improved serum stability profiles. [18F]-C-SNAT4 cellular update was elevated in NCI-H460 cells in a time- and dose-dependent manner, which correlated well with cell death. A significant increase in [18F]-C-SNAT4 uptake was measured in NCI-H460 tumor xenografts in mice. In contrast, a rapid clearance of [18F]-C-SNAT4 was observed in drug-resistant NCI-H1299 in vitro and in tumor xenografts. Moreover, in BALB/C mice bearing murine colon cancer CT26 tumor xenografts receiving checkpoint inhibitors, [18F]-C-SNAT4 showed its ability for monitoring immunotherapy-induced apoptosis and reporting treatment-responding mice from non-responding.
Conclusion
The uptake of [18F]-C-SNAT4 in tumors received chemotherapy and immunotherapy is positively correlated with the tumor apoptotic level and the treatment efficacy. [18F]-C-SNAT4 PET imaging can monitor tumor response to two different treatment modalities and predict the therapeutic efficacy in preclinical mouse models.






Similar content being viewed by others
Data availability
Original data are available on request.
References
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Kummar S, Gutierrez M, Doroshow JH, Murgo AJ. Drug development in oncology: classical cytotoxics and molecularly targeted agents. Br J Clin Pharmacol. 2006;62:15–26.
Fox E, Curt GA, Balis FM. Clinical trial design for target-based therapy. Oncologist. 2002;7:401–9.
Weber WA. Assessing tumor response to therapy. J Nucl Med. 2009;50:1S–10S.
Rankin S. PET/CT for staging and monitoring non small cell lung cancer. Cancer Imaging. 2008;18:S27–31.
Irkle A, Vesey AT, Lewis DY, et al. Identifying active vascular microcalcification by 18F-sodium fluoride positron emission tomography. Nat Commun. 2015;6:7495.
Weber WA, Petersen V, Schmidt B, et al. Positron emission tomography in non-small-cell lung cancer: prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Oncol. 2003;21:2651–7.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50:122S–50S.
Ertay T, Sencan Eren M, Karaman M, Oktay G, Durak H. 18F-FDG-PET/CT in initiation and progression of inflammation and infection. Mol Imaging Radionucl Ther. 2017;26:47–52.
Li XF, Du Y, Ma Y, Postel GC, Civelek AC. 18F-fluorodeoxyglucose uptake and tumor hypoxia: revisit 18F-fluorodeoxyglucose in oncology application. Transl Oncol. 2014;7:240–7.
Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2014;2014:150845.
Blankenberg FG. In vivo detection of apoptosis. J Nucl Med. 2008;49:81S–95S.
Brindle K. New approaches for imaging tumour responses to treatment. Nat Rev Cancer. 2008;8:94–107.
Zhang D, Jin Q, Jiang C, Gao M, Ni Y, Zhang J. Imaging cell death: focus on early evaluation of tumor response to therapy. Bioconjug Chem. 2020;31:1025–51.
Murakami Y, Takamatsu H, Taki J, et al. 18F-labelled annexin V: a PET tracer for apoptosis imaging. Eur J Nucl Med Mol Imaging. 2004;31:469–74.
Neves AA, Brindle KM. Imaging cell death. J Nucl Med. 2014;55:1–4.
Reshef A, Shirvan A, Akselrod-Ballin A, Wall A, Ziv I. Small-molecule biomarkers for clinical PET imaging of apoptosis. J Nucl Med. 2010;51:837–40.
Grimberg H, Levin G, Shirvan A, et al. Monitoring of tumor response to chemotherapy in vivo by a novel small-molecule detector of apoptosis. Apoptosis. 2009;14:257–67.
Hoglund J, Shirvan A, Antoni G, et al. 18F-ML-10, a PET tracer for apoptosis: first human study. J Nucl Med. 2011;52:720–5.
Madar I, Huang Y, Ravert H, et al. Detection and quantification of the evolution dynamics of apoptosis using the PET voltage sensor 18F-fluorobenzyl triphenyl phosphonium. J Nucl Med. 2009;50:774–80.
Madar I, Ravert H, Nelkin B, et al. Characterization of membrane potential-dependent uptake of the novel PET tracer 18F-fluorobenzyl triphenylphosphonium cation. Eur J Nucl Med Mol Imaging. 2007;34:2057–65.
Alan GP, Reiner UJ. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104.
Witney TH, Fortt R, Aboagye EO. Preclinical assessment of carboplatin treatment efficacy in lung cancer by 18F-ICMT-11-positron emission tomography. PLoS One. 2014;9:e91694–4.
Nguyen Q-D, Lavdas I, Gubbins J, et al. Temporal and spatial evolution of therapy-induced tumor apoptosis detected by caspase-3–selective molecular imaging. Clin Cancer Res. 2013;19:3914.
Nguyen Q-D, Smith G, Glaser M, Perumal M, Arstad E, Aboagye EO. Positron emission tomography imaging of drug-induced tumor apoptosis with a caspase-3/7 specific [18F]-labeled isatin sulfonamide. Proc Natl Acad Sci U S A. 2009;106:16375–80.
Challapalli A, Kenny LM, Hallett WA, et al. 18F-ICMT-11, a caspase-3-specific PET tracer for apoptosis: biodistribution and radiation dosimetry. J Nucl Med. 2013;54:1551–6.
Chen DL, Zhou D, Chu W, et al. Radiolabeled isatin binding to caspase-3 activation induced by anti-Fas antibody. Nucl Med Biol. 2012;39:137–44.
Methot N, Vaillancourt JP, Huang J, et al. A caspase active site probe reveals high fractional inhibition needed to block DNA fragmentation. J Biol Chem. 2004;279:27905–14.
Villa P, Kaufmann SH, Earnshaw WC. Caspases and caspase inhibitors. Trends Biochem Sci. 1997;22:388–93.
Doss M, Kolb HC, Walsh JC, et al. Biodistribution and radiation dosimetry of 18F-CP-18, a potential apoptosis imaging agent, as determined from PET/CT scans in healthy volunteers. J Nucl Med. 2013;54:2087–92.
Shen B, Jeon J, Palner M, et al. Positron emission tomography imaging of drug-induced tumor apoptosis with a caspase-triggered nanoaggregation probe. Angew Chem Int Ed Engl. 2013;52:10511–4.
Ye D, Shuhendler AJ, Cui L, et al. Bioorthogonal cyclization-mediated in situ self-assembly of small-molecule probes for imaging caspase activity in vivo. Nat Chem. 2014;6:519–26.
Palner M, Shen B, Jeon J, Lin J, Chin FT, Rao J. Preclinical kinetic analysis of the caspase-3/7 PET tracer 18F-C-SNAT: quantifying the changes in blood flow and tumor retention after chemotherapy. J Nucl Med. 2015;56:1415–21.
Hartshorn CM, Bradbury MS, Lanza GM, et al. Nanotechnology strategies to advance outcomes in clinical cancer care. ACS Nano. 2018;12:24–43.
Witney TH, Hoehne A, Reeves RE, et al. A systematic comparison of 18F-C-SNAT to established radiotracer imaging agents for the detection of tumor response to treatment. Clin Cancer Res. 2015;21:3896–905.
Chen Z, Chen M, Cheng Y, et al. Exploring the condensation reaction between aromatic nitriles and amino thiols to optimize in situ nanoparticle formation for the imaging of proteases and glycosidases in cells. Angew Chem Int Ed Engl. 2020;59:3272–9.
Niitani H, Kobayashi K. Cisplatin/carboplatin therapy in non-small cell lung cancer. Oncology. 1992;49:51–6.
Xu XM, Zhang Y, Qu D, et al. Combined anticancer activity of osthole and cisplatin in NCI-H460 lung cancer cells in vitro. Exp Ther Med. 2013;5:707–10.
Hientz K, Mohr A, Bhakta-Guha D, Efferth T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget. 2017;8:8921–46.
Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–10.
Cai Y, Yan X, Zhang GQ, Zhao WH, Jiao GY. The predictive value of ERCC1 and p53 for the effect of panobinostat and cisplatin combination treatment in NSCLC. Oncotarget. 2015;6:18997–9005.
Krekorian M, Fruhwirth GO, Srinivas M, et al. Imaging of T-cells and their responses during anti-cancer immunotherapy. Theranostics. 2019;9:7924–47.
Sachpekidis C, Anwar H, Winkler J, et al. The role of interim (18)F-FDG PET/CT in prediction of response to ipilimumab treatment in metastatic melanoma. Eur J Nucl Med Mol Imaging. 2018;45:1289–96.
Anwar H, Sachpekidis C, Winkler J, et al. Absolute number of new lesions on (18)F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur J Nucl Med Mol Imaging. 2018;45:376–83.
Sachpekidis C, Kopp-Schneider A, Pan L, et al. Interim [18F] FDG PET/CT can predict response to anti-PD-1 treatment in metastatic melanoma. Eur J Nucl Med Mol Imaging. 2020.
Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20.
Park HJ, Kim KW, Pyo J, et al. Incidence of pseudoprogression during immune checkpoint inhibitor therapy for solid tumors: a systematic review and meta-analysis. Radiology. 2020;297:87–96.
Dimitrakopoulou-Strauss A. Monitoring of patients with metastatic melanoma treated with immune checkpoint inhibitors using PET-CT. Cancer Immunol Immunother. 2019;68:813–22.
Metkar SS, Wang B, Ebbs ML, et al. Granzyme B activates procaspase-3 which signals a mitochondrial amplification loop for maximal apoptosis. J Cell Biol. 2003;160:875–85.
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–91.
Stein A, Folprecht G. Immunotherapy of colon cancer. Oncol Res Treat. 2018;41:282–5.
Larimer BM, Wehrenberg-Klee E, Dubois F, et al. Granzyme B PET imaging as a predictive biomarker of immunotherapy response. Cancer Res. 2017;77:2318–27.
Rosenblum D, Joshi N, Tao W, Karp JM, Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun. 2018;9:1410.
Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20–37.
Qiu L, Li X, Lv G, et al. Radiofluorinated smart probes for noninvasive PET imaging of legumain activity in living subjects. Anal Chem. 2020;92:11627–34.
Wang S, Gao D, Li K, et al. Radiopharmacological evaluation of a caspase-3 responsive probe with optimized pharmacokinetics for PET imaging of tumor apoptosis. Org Biomol Chem. 2020;18:3512–21.
Qiu L, Wang W, Li K, et al. Rational design of caspase-responsive smart molecular probe for positron emission tomography imaging of drug-induced apoptosis. Theranostics. 2019;9:6962–75.
Liu Y, Miao Q, Zou P, et al. Enzyme-controlled intracellular self-assembly of 18F nanoparticles for enhanced microPET imaging of tumor. Theranostics. 2015;5:1058–67.
Chen Z, Chen M, Zhou K, Rao J. Pre-targeted iaging of protease activity through in situ assembly of nnoparticles. Angew Chem Int Ed Engl. 2020;59:7864–70.
Lorke DE, Krüger M, Buchert R, Bohuslavizki KH, Clausen M, Schumacher U. In vitro and in vivo tracer characteristics of an established multidrug-resistant human colon cancer cell line. J Nucl Med. 2001;42:646–54.
Muhammad IF, Borne Y, Melander O, et al. FADD (Fas-asociated potein wth dath dmain), cspase-3, and caspase-8 and icidence of ichemic stroke. Stroke. 2018;49:2224–6.
Drakopanagiotakis F, Xifteri A, Polychronopoulos V, Bouros D. Apoptosis in lung injury and fibrosis. Eur Respir J. 2008;32:1631–8.
Cippa PE, Fehr T. Pharmacological modulation of cell death in organ transplantation. Transpl Int. 2017;30:851–9.
Acknowledgements
This work was supported by a grant from National Cancer Institute (NCI) Stanford CCNE-TD (U54CA199075). M.C. acknowledges the support by the NCI-funded Stanford Cancer Translational Nanotechnology Training grant (T32CA196585). L.C. acknowledges the support by the Office of the Assistant Secretary of Defense for Health Affairs through the Breast Cancer Research Program (BCRP) under Award No. (W81XWH-18-1-0591). The authors acknowledge the use of Stanford Center for Innovation in In-Vivo Imaging (SCI3) Core Facility, Stanford Neuroscience Microscopy Service (Supported by NIH NS069375), and Stanford Nano Shared Facilities (SNSF) (Supported by the National Science Foundation under award ECCS-1542152). We thank Emily Johnson and Michelle James for their technical assistance in the PET experiments, and Xue Wu for her assistance in the biodistribution experiments.
Funding
This work was supported by a grant from National Cancer Institute (NCI) Stanford CCNE-TD (U54CA199075). M.C. acknowledges the support by the NCI-funded Stanford Cancer Translational Nanotechnology Training grant (T32CA196585). L.C. acknowledges the support by the Office of the Assistant Secretary of Defense for Health Affairs through the Breast Cancer Research Program (BCRP) under Award No. (W81XWH-18-1-0591). The authors acknowledge the use of Stanford Center for Innovation in In-Vivo Imaging (SCI3) Core Facility, Stanford Neuroscience Microscopy Service (Supported by NIH NS069375), and Stanford Nano Shared Facilities (SNSF) (Supported by the National Science Foundation under award ECCS-1542152).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Chen, Z. Chen, J. Xie, and J. Rao are inventors of a patent application filed by Stanford University covering part of the information contained in the paper. No other potential conflicts of interest relevant to this article exist.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Preclinical Imaging.
Rights and permissions
About this article
Cite this article
Chen, M., Chen, Z., Castillo, J.B. et al. [18F]-C-SNAT4: an improved caspase-3-sensitive nanoaggregation PET tracer for imaging of tumor responses to chemo- and immunotherapies. Eur J Nucl Med Mol Imaging 48, 3386–3399 (2021). https://doi.org/10.1007/s00259-021-05297-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05297-0