Abstract
Purpose
Mitochondrial dysfunction has been attributed a critical role in the etiology and pathogenesis of numerous diseases, and is manifested by alterations of the organelle’s membrane potential (Δψm). This suggests that Δψm measurement can be highly useful for diagnostic purposes. In the current study, we characterized the capability of the novel PET agent 18F-fluorobenzyl triphenylphosphonium (18F-FBnTP) to assess Δψm, compared with the well-established voltage sensor 3H-tetraphenylphosphonium (3H-TPP).
Methods
18F-FBnTP and 3H-TPP uptake under conditions known to alter Δψm and plasma membrane potential (Δψp) was assayed in the H345 lung carcinoma cell line. 18F-FBnTP biodistribution was assessed in CD1 mice using dynamic PET and ex vivo gamma well counting.
Results
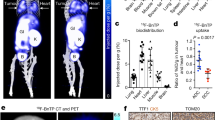
18F-FBnTP and 3H-TPP demonstrated similar uptake kinetics and plateau concentrations in H345 cells. Stepwise membrane depolarization resulted in a linear decrease in 18F-FBnTP cellular uptake, with a slope (−0.58±0.06) and correlation coefficient (0.94±0.07) similar (p>0.17) to those measured for 3H-TPP (−0.63±0.06 and 0.96±0.05, respectively). Selective collapse of Δψm caused a substantial decrease in cellular uptake for 18F-FBnTP (81.6±8.1%) and 3H-TPP (85.4±6.7%), compared with control. Exposure to the proapoptotic staurosporine, known to collapse Δψm, resulted in a decrease of 68.7±10.1% and 71.5±8.4% in 18F-FBnTP and 3H-TPP cellular uptake, respectively. 18F-FBnTP accumulated mainly in kidney, heart and liver.
Conclusion
18F-FBnTP is a mitochondria-targeting PET radiopharmaceutical responsive to alterations in membrane potential with voltage-dependent performance similar to that of 3H-TPP. 18F-FBnTP is a promising new voltage sensor for detection of physiological and pathological processes associated with mitochondrial dysfunction, such as apoptosis, using PET.







Similar content being viewed by others
References
Duchen MR. Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol Aspects Med 2004;25:365–451.
Nicholls DG. Mitochondrial dysfunction and glutamate excitotoxicity studied in primary neuronal cultures. Curr Mol Med 2004;4:149–77.
Liberman EA, Skulachev VP. Conversion of biomembrane-produced energy into electric form. IV. General discussion. Biochim Biophys Acta 1970;216:30–42.
Murphy MP, Smith RA. Drug delivery to mitochondria: the key to mitochondrial medicine. Adv Drug Deliv Rev 2000;41:235–50.
Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science 2004;305:626–9.
Susin SA, Zamzami N, Kroemer G. Mitochondria as regulators of apoptosis: doubt no more. Biochim Biophys Acta 1998;1366:151–65.
Grinius LL, Jasaitis AA, Kadziauskas YP, Liberman EA, Skulachev VP, Topali VP, et al. Conversion of biomembrane-produced energy into electric form. I. Submitochondrial particles. Biochim Biophys Acta 1970;216:1–12.
Ravert HT, Madar I, Dannals RF. Radiosynthesis of 3-[18F]-fluoropropyl and 4-[18F]-fluorobenzyl triarylphosphonium ions. J Label Compd Radiopharm 2004;47:469–76.
Madar I, Ravert RT, Du Y, Hilton J, Dannels RF, Frost JJ, et al. Characterization of uptake of the new PET imaging compound [18F]fluorobenzyl triphenyl phosphonium in dog myocardium. J Nucl Med 2006;47:1359–66.
Piwnica-Worms D, Kronauge JF, Chiu ML. Uptake and retention of hexakis (2-methoxyisobutyl isonitrile) technetium(I) in cultured chick myocardial cells. Mitochondrial and plasma membrane potential dependence. Circulation 1990;82:1826–38.
Pivovarova NB, Nguyen HV, Winters CA, Brantner CA, Smith CL, Andrews SB. Excitotoxic calcium overload in a subpopulation of mitochondria triggers delayed death in hippocampal neurons. J Neurosci 2004;24:5611–22.
Wang Y, Seidel J, Tsui BM, Vaquero JJ, Pomper MG. Performance evaluation of the GE healthcare eXplore VISTA dual-ring small-animal PET scanner. J Nucl Med 2006;47:1891–900.
Freedman JC, Laris PC. Electrophysiology of cells and organelles: studies with optical potentiometric indicators. Int Rev Cytol Suppl 1981;12:177–246.
Pancrazio JJ, Tabbara IA, Kim YI. Voltage-activated K+ conductance and cell proliferation in small-cell lung cancer. Anticancer Res 1993;13:1231–4.
Wonderlin WF, Woodfork KA, Strobl JS. Changes in membrane potential during the progression of MCF-7 human mammary tumor cells through the cell cycle. J Cell Physiol 1995;165:177–85.
Hanstein WG. Uncoupling of oxidative phosphorylation. Biochim Biophys Acta 1976;456:129–48.
Lichtshtein D, Dunlop K, Kaback HR, Blume AJ. Mechanism of monensin-induced hyperpolarization of neuroblastoma glioma hybrid NG108-15. Proc Natl Acad Sci U S A 1979;76:2580–4.
O’Brien TM, Carlson RM, Oliveira PJ, Wallace KB. Esterification prevents induction of the mitochondrial permeability transition by N-acetyl perfluorooctane sulfonamides. Chem Res Toxicol 2006;19:1305–12.
Stephans SE, Miller GW, Levey AI, Greenamyre JT. Acute mitochondrial and chronic toxicological effects of 1-methyl-4-phenylpyridinium in human neuroblastoma cells. Neurotoxicology 2002;23:569–80.
Cassarino DS, Swerdlow RH, Parks JK, Parker WD Jr, Bennett JP Jr. Cyclosporin A increases resting mitochondrial membrane potential in SY5Y cells and reverses the depressed mitochondrial membrane potential of Alzheimer’s disease cybrids. Biochem Biophys Res Commun 1998;248:168–73.
Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett 1997;411:77–82.
Appleby RD, Porteous WK, Hughes G, James AM, Shannon D, Wei YH, et al. Quantitation and origin of the mitochondrial membrane potential in human cells lacking mitochondrial DNA. Eur J Biochem 1999;262:108–16.
Rottenberg H. Membrane potential and surface potential in mitochondria: uptake and binding of lipophilic cations. J Membr Biol 1984;81:127–38.
Ross MF, Kelso GF, Blaikie FH, James AM, Cocheme HM, Filipovska A, et al. Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochemistry (Mosc) 2005;70:222–30.
Cheng Z, Winant RC, Gambhir SS. A new strategy to screen molecular imaging probe uptake in cell culture without radiolabeling using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Nucl Med 2005;46:878–86.
Davis S, Weiss MJ, Wong JR, Lampidis TJ, Chen LB. Mitochondrial and plasma membrane potentials cause unusual accumulation and retention of rhodamine 123 by human breast adenocarcinoma-derived MCF-7 cells. J Biol Chem 1985;260:13844–50.
Nicholls DG, Ward MW. Mitochondrial membrane potential and neuronal glutamate excitotoxicity: mortality and millivolts. Trends Neurosci 2000;23:166–74.
Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J 1998;17:1675–87.
Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther 2005;4:139–63.
Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes 2002;51:159–67.
Scarlett JL, Sheard PW, Hughes G, Ledgerwood EC, Ku HH, Murphy MP. Changes in mitochondrial membrane potential during staurosporine-induced apoptosis in Jurkat cells. FEBS Lett 2000;475:267–72.
Joseph B, Marchetti P, Formstecher P, Kroemer G, Lewensohn R, Zhivotovsky B. Mitochondrial dysfunction is an essential step for killing of non-small cell lung carcinomas resistant to conventional treatment. Oncogene 2002;21:65–77.
Crow MT, Mani K, Nam YJ, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res 2004;95:957–70.
Kaushal GP, Basnakian AG, Shah SV. Apoptotic pathways in ischemic acute renal failure. Kidney Int 2004;66:500–6.
Hinescu ME. Cardiac apoptosis: from organ failure to allograft rejection. J Cell Mol Med 2001;5:143–52.
Boersma HH, Kietselaer BL, Stolk LM, Bennaghmouch A, Hofstra L, Narula J, et al. Past, present, and future of annexin A5: from protein discovery to clinical applications. J Nucl Med 2005;46:2035–50.
Lahorte CM, Vanderheyden JL, Steinmetz N, Van de Wiele C, Dierckx RA, Slegers G. Apoptosis-detecting radioligands: current state of the art and future perspectives. Eur J Nucl Med Mol Imaging 2004;31:887–919.
Damianovich M, Ziv I, Heyman SN, Rosen S, Shina A, Kidron D, et al. ApoSense: a novel technology for functional molecular imaging of cell death in models of acute renal tubular necrosis. Eur J Nucl Med Mol Imaging 2006;33:281–91.
Aloya R, Shirvan A, Grimberg H, Reshef A, Levin G, Kidron D, et al. Molecular imaging of cell death in vivo by a novel small molecule probe. Apoptosis 2006;11:2089–101.
Chen LB. Mitochondrial membrane potential in living cells. Annu Rev Cell Biol 1988;4:155–81.
Madar I, Weiss L, Izbicki G. Preferential accumulation of 3H-tetraphenylphosphonium in non-small cell lung carcinoma in mice: comparison with99mTc-MIBI. J Nucl Med 2002;43:234–8.
Madar I, Anderson JH, Szabo Z, Scheffel U, Kao PF, Ravert HT, et al. Enhanced uptake of [11C]TPMP in canine brain tumor: a PET study. J Nucl Med 1999;40:1180–5.
Min JJ, Biswal S, Deroose C, Gambhir SS. Tetraphenylphosphonium as a novel molecular probe for imaging tumors. J Nucl Med 2004;45:636–43.
Madar I, Scheffel U, Ravert HT, Dannals RF, Frost JJ. Differential distinction between tumor and inflammation using the voltage indicator [F-18]p-fluorobenzylriphenyl phosphonium (F-18]p-FBnTP): comparison with [F-18]FDG. J Nucl Med 2003;44:368.
Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp Cell Res 2000;256:42–9.
Mow BM, Blajeski AL, Chandra J, Kaufmann SH. Apoptosis and the response to anticancer therapy. Curr Opin Oncol 2001;13:453–62.
Gros P, Talbot F, Tang-Wai D, Bibi E, Kaback HR. Lipophilic cations: a group of model substrates for the multidrug resistance transporter. Biochemistry 1992;31:1992–8.
Bibi E, Gros P, Kaback HR. Functional expression of mouse mdr1 inEscherichia coli. Proc Natl Acad Sci U S A 1993;90:9209–13.
Acknowledgements
We thank Dr. Hagai Rottenberg for fruitful discussions and James Fox for the acquisition of the PET scans. This work was supported in part by R24 CA92871.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madar, I., Ravert, H., Nelkin, B. et al. Characterization of membrane potential-dependent uptake of the novel PET tracer 18F-fluorobenzyl triphenylphosphonium cation. Eur J Nucl Med Mol Imaging 34, 2057–2065 (2007). https://doi.org/10.1007/s00259-007-0500-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-007-0500-8