Abstract
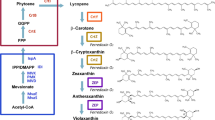
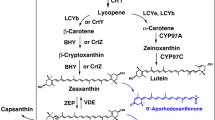
Violaxanthin is biosynthesized from zeaxanthin with zeaxanthin epoxidase (ZEP) by way of antheraxanthin only in photosynthetic eukaryotes including higher plants and involved in the xanthophyll cycle to eliminate excessive light energy. Violaxanthin and antheraxanthin have commercially been unavailable, in contrast to commercial production of other carotenoids contained in higher plants, e.g., lycopene, β-carotene, lutein, zeaxanthin, β-cryptoxanthin, and capsanthin. One of the reasons is considered that resource plants or other resource organisms do not exist for enabling efficient supply of the epoxy-carotenoids, which are expected to be produced through (metabolic) pathway engineering with heterologous microbial hosts such as Escherichia coli and Saccharomyces cerevisiae. In this Mini-Review, we show heterologous production of violaxanthin with the two microorganisms that have exhibited significant advances these days. We further describe natural function and occurrence, and biosynthesis involving violaxanthin, antheraxanthin, and their derivatives that include auroxanthin and mutatoxanthin.
Key points
• A comprehensive review on epoxy-carotenoids violaxanthin and antheraxanthin.
• Pathway engineering for the epoxy-carotenoids in heterologous microbes.
• Our new findings on violaxanthin production with the budding yeast.





Similar content being viewed by others
References
Alcaíno J, Baeza M, Cifuentes V (2016) Carotenoid distribution in nature. In: Stange C (ed) Carotenoids in Nature. Subcellular Biochemistry, vol 79. Springer, Cham, pp 3–33. https://doi.org/10.1007/978-3-319-39126-7_1
Alper H, Stephanopoulos G (2008) Uncovering the gene knockout landscape for improved lycopene production in E. coli. Appl Microbiol Biotechnol 78:801–810
Araki M, Kaku N, Harada M, Ando Y, Yamaguchi R, Shindo K (2016) Production of auroxanthins from violaxanthin and 9-cis-violaxanthin by acidic treatment, and the antioxidant activities of violaxanthin, 9-cis-violaxanthin, and auroxanthins. J Agric Food Chem 64:9352–9355
Baneyx F (1999) Recombinant protein expression in Escherichia coli. Curr Opin Biotechnol 10:411–421
Bartley GE, Scolnik PA, Beyer P (1999) Two Arabidopsis thaliana carotene desaturases, phytoene desaturase and ζ-carotene desaturase, expressed in Escherichia coli, catalyze a poly-cis pathway to yield pro-lycopene. FEBS J 259:396–403
Bongers M, Chrysanthopoulos PK, Behrendorff JBYH, Hodson MP, Vickers CE, Nielsen LK (2015) Systems analysis of methylerythritol-phosphate pathway flux in E. coli: insights into the role of oxidative stress and the validity of lycopene as an isoprenoid reporter metabolite. Microb Cell Factories 14:193
Bouvier F, d’Harlingue A, Hugueney P, Marin E, Marion-Poll A, Camara B (1996) Xanthophyll Biosynthesis—cloning, expression, functional reconstitution, and regulation of β-cyclohexenyl carotenoid epoxidase from pepper (Capsicum annuum). J Biol Chem 271:28861–28867
Britton G, Liaaen-Jensen S, Pfander H (2004) Carotenoids Handbook. Birkhäuser Verlag, Basel, Boston, Berlin
Buch K, Stransky H, Hager A (1995) FAD is a further essential cofactor of the NAD(P)H and O2-dependent zeaxanthin-epoxidase. FEBS Lett 376:45–48
Cataldo VF, Arenas N, Salgado V, Camilo C, Ibáñez F, Agosin E (2020) Heterologous production of the epoxycarotenoid violaxanthin in Saccharomyces cerevisiae. Metab Eng 59:53–63
Chae HS, Kim KH, Kim SC, Lee PC (2010) Strain-dependent carotenoid productions in metabolically engineered Escherichia coli. Appl Biochem Biotechnol 162:2333–2344
Chen Y, Xiao W, Wang Y, Liu H, Li X, Yuan Y (2016) Lycopene overproduction in Saccharomyces cerevisiae through combining pathway engineering with host engineering. Microb Cell Factories 15:113
Ciccone MM, Cortese F, Gesualdo M, Carbonara S, Zito A, Ricci G, Pascalis FD, Scicchitano P, Riccioni G (2013) Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediat Inflamm 2013:782137–782111. https://doi.org/10.1155/2013/782137
Dautermann O, Lyska D, Andersen-Ranberg J, Becker M, Fröhlich-Nowoisky J, Gartmann H, Krämer LC, Mayr K, Pieper D, Rij LM, Wipf HML, Niyogi KK, Lohr M (2020) An algal enzyme required for biosynthesis of the most abundant marine carotenoids. Sci Adv 6(10):eaaw9183. https://doi.org/10.1126/sciadv.aaw9183
Deli J, Ösz E (2004) Carotenoid 5,6-, 5,8-, and 3,6-epoxides. Issue in Honor of Prof. Sándor Antus. Arkivoc (vii):150–168
Demmig-Adams B, Adams WW III (1996) The role of the xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci 1:21–26
Eilers U, Dietzel L, Breitenbach J, Büchel C, Sandmann G (2016) Identification of genes coding for functional zeaxanthin epoxidases in the diatom Paeodactylum tricornutum. J Plant Physiol 192:64–70
Fiedor J, Burda K (2014) Potential role of carotenoids as antioxidants in human health and disease. Nutrients 6:466–488
Frank HA, Cogdell RJ (1993) The photochemistry and function of carotenoids in photosynthesis. In: Young AJ, Britton G (eds) Carotenoids in Photosynthesis. Springer, Dordrecht, pp 252–326
Fraser PD, Bramley PM (2004) The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res 43:228–265
Fujisaki S, Hara H, Nishimura Y, Horiuchi K, Nishino T (1990) Cloning and nucleotide sequence of the ispA gene responsible for farnesyl diphosphate synthase activity in Escherichia coli. J Biochem 108:995–1000
Fujisawa Y, Hashimoto K, Manabe K, Maoka T (2002) Structures of tobiraxanthin A1, A2, A3, B, C and D, new carotenoids from the seeds of Pittosporum tobira. Tetrahedron Lett 43:4385–4388
Furubayashi M, Kubo A, Takemura M, Otani Y, Maoka T, Terada Y, Yaoi K, Ohdan K, Misawa N, Mitani Y (2021) Capsanthin production in Escherichia coli by overexpression of capsanthin/capsorubin synthase from Capsicum annuum. J Agric Food Chem 69(17):5076–5085. https://doi.org/10.1021/acs.jafc.1c00083
Giuliano G (2014) Plant carotenoids: genomics meets multi-gene engineering. Curr Opin Plant Biol 19:111–117
Goodwin TW, Britton G (1988) Distribution and analysis of carotenoids. In: Goodwin TW (ed) Plant pigments. Academic Press, pp 61–132
Harada H, Yu F, Okamoto S, Kuzuyama T, Utsumi R, Misawa N (2009) Efficient synthesis of functional isoprenoids from acetoacetate through metabolic pathway-engineered Escherichia coli. Appl Microbiol Biotechnol 81:915–925
Kajiwara S, Fraser PD, Kondo K, Misawa N (1997) Expression of an exogenous isopentenyl diphosphate isomerase gene enhances isoprenoid biosynthesis in Escherichia coli. Biochem J 324:421–426
Kaneda K, Kuzuyama T, Takagi M, Hayakawa Y, Seto H (2001) An unusual isopentenyl diphosphate isomerase found in the mevalonate pathway gene cluster from Streptomyces sp. strain CL190. Proc Natl Acad Sci 98:932–937
Kim J, DellaPenna D (2006) Defining the primary route for lutein synthesis in plants: the role of Arabidopsis carotenoid β-ring hydroxylase CYP97A3. Proc Natl Acad Sci 103:3474–3479
Leber R, Landl K, Zinser E, Ahorn H, Spok A, Kohlwein SD, Turnowsky F, Daum G (1998) Dual localization of squalene epoxidase, Erg1p, in yeast reflects a relationship between the endoplasmic reticulum and lipid particles. Mol Biol Cell 9:375–386
Lee P, Schmidt-Dannert C (2002) Metabolic engineering towards biotechnological production of carotenoids in microorganisms. Appl Microbiol Biotechnol 60:1–11
Lewis MJ, Sweet DJ, Pelham HRB (1990) The ERD2 gene determines the specificity of the luminal ER protein retention system. Cell 61:1359–1363
Li XR, Tian GQ, Shen HJ, Liu JZ (2015) Metabolic engineering of Escherichia coli to produce zeaxanthin. J Ind Mcrobiol Biotechnol 42:627–636
Linden H, Misawa N, Saito T, Sandmann G (1994) A novel carotenoid biosynthesis gene coding for ζ-carotene desaturase: functional expression, sequence and phylogenetic origin. Plant Mol Biol 24:369–379
López J, Cataldo VF, Peña M, Saa PA, Saitua F, Ibaceta M, Agosin E (2019) Build your bioprocess on a solid strain—β-carotene production in recombinant Saccharomyces cerevisiae. Front Bioeng Biotechnol 7:171
Ma T, Shi B, Ye Z, Li X, Liu M, Chen Y, Xia J, Nielsen J, Deng Z, Liu T (2019) Lipid engineering combined with systematic metabolic engineering of Saccharomyces cerevisiae for high-yield production of lycopene. Metab Eng 52:134–142
Maoka T, Fujisawa Y, Hashimoto K, Akimoto N (2006) 5-Hydroxy-seco-carotenoids from Pittosporum tobira. Phytochem 67:2120–2125
Marz U (2015) The global market for carotenoids. A BCC Research Food & Beverage Report, July 2015, Report ID:FOD025E, bcc Research
Misawa N (2010) Carotenoids. In: Mander L, Lui HW (eds) Comprehensive Natural Products II Chemistry and Biology, vol 1. Elsevier, Oxford, pp 733–753
Misawa N (2011) Pathway engineering for functional isoprenoids. Curr Opin Biotechnol 22:627–633
Misawa N (ed) (2021) Carotenoids: Biosynthetic and Biofunctional Approaches. In: Adv Exp Med Biol, vol 1261. Springer, Singapore
Misawa N, Nakagawa M, Kobayashi K, Yamano S, Izawa Y, Nakamura K, Harashima K (1990) Elucidation of the Erwinia uredovora carotenoid biosynthetic pathway by functional analysis of gene products expressed in Escherichia coli. J Bacteriol 172:6704–6712
Misawa N, Satomi Y, Kondo K, Yokoyama A, Kajiwara S, Saito T, Ohtani T, Miki W (1995) Structure and functional analysis of a marine bacterial carotenoid biosynthesis gene cluster and astaxanthin biosynthetic pathway proposed at the gene level. J Bacteriol 177:6575–6584
Moise AR, Al-Babili S, Wutzel ET (2014) Mechanistic aspects of carotenoid biosynthesis. Chem Rev 114:164–193
Mori A, Hara S, Sugahara T, Kojima T, Iwasaki Y, Kawarasaki Y, Sahara T, Ohgiya S, Nakano H (2015) Signal peptide optimization tool for the secretion of recombinant protein from Saccharomyces cerevisiae. J Biosci Bioeng 120:518–525
Nishino H, Tokuda H, Satomi Y, Masuda M, Bu P, Onozuka M, Yamaguchi S, Okuda Y, Takayasu J, Tsuruta J, Okuda M, Ichiishi E, Murakoshi M, Kato T, Misawa N, Narisawa T, Takasuka N, Yano M (1999) Cancer prevention by carotenoids. Pure Appl Chem 71:2273–2278
Ohmiya A (2011) Diversity of carotenoid composition in flower petals. JARQ 45:163–171
Quinlan RF, Jaradat TT, Wurtzel ET (2007) Escherichia coli as a platform for functional expression of plant P450 carotene hydroxylases. Arch Biochem Biophys 458:146–157
Reyes LH, Kao KC (2018) Growth-coupled carotenoids production using adaptive laboratory evolution. Methods Mol Biol Clifton N J 1671:319–330
Reyes LH, Gomez JM, Kao KC (2014) Improving carotenoids production in yeast via adaptive laboratory evolution. Metab Eng 21:26–33
Satoh T, Horie M, Watanabe H, Tsuchiya Y, Kamei T (1993) Enzymatic properties of squalene epoxidase from Saccharomyces cerevisiae. Biol Pharm Bull 16:349–352
Schaller S, Wilhelm C, Strzałka K, Goss R (2012) Investigating the interaction between the violaxanthin cycle enzyme zeaxanthin epoxidase and the thylakoid membrane. J Photochem Photobiol B Biol 114:119–125
Shimode S, Miyata K, Araki M, Shindo K (2018) Antioxidant activities of the antheraxanthin-related carotenoids, antheraxanthin, 9-cis-antheraxanthin, and mutatoxanthins. Oleo Sci 67:977–981
Shimode S, Miyata K, Takemura M, Shimada H, Misawa N, Shindo K (2020) A new carotenoid, 6′-hydroxy-3′-didehydro-lutein, produced by recombinant Escherichia coli that expresses the violaxanthin biosynthesis and chaperone AtCYO1 genes—its structure and antioxidant activities. Phytochem Lett 35:113–118
Sikorski RS, Hieter P (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19–27
Stahl W, Site H (2004) Bioactivity and protective effects of natural carotenoids. Biochim Biophys Acta 1740:101–107
Takaichi S (2011) Carotenoids in algae: distributions, biosynthesis and functions. Mar Drugs 9:1101–1118
Takemura M, Maoka T, Misawa N (2014) Carotenoid analysis of a liverwort Marchantia polymorpha and functional identification of its lycopene β- and ε-cyclase genes. Plant Cell Physiol 55:194–200
Takemura M, Maoka T, Misawa N (2015a) Biosynthetic routes of hydroxylated carotenoids (xanthophylls) in Marchantia polymorpha (liverwort), and production of novel and rare xanthophylls through pathway engineering in Escherichia coli. Planta 241:699–710
Takemura M, Maoka T, Osawa A, Higashinaka H, Shimada H, Shindo K, Misawa N (2015b) (6E) and (6Z)-9’-Aporhodoxanthinone, novel carotenoids produced in zeaxanthin-synthesizing-Escherichia coli by redox stress. Tetrahedron Lett 56:6063–6065
Takemura M, Kubo A, Higuchi Y, Maoka T, Sahara T, Yaoi K, Ohdan K, Umeno D, Misawa N (2019) Pathway engineering for efficient biosynthesis of violaxanthin in Escherichia. coli. Appl Microbiol Biotechnol 103:9393–9399
Toniolo P, Van Kappel AL, Akhmedkhanov A, Ferrari P, Kato I, Shore RE, Riboli E (2001) Serum carotenoids and breast cancer. Am J Epidemiol 153:1142–1147
Ukibe K, Hashida K, Yoshida N, Takagi H (2009) Metabolic engineering of Saccharomyces cerevisiae for astaxanthin production and oxidative stress tolerance. Appl Environ Microbiol 75:7205–7211
Verwaal R, Wang J, Meijnen J-P, Visser H, Sandmann G, van den Berg JA, van Ooyen AJJ (2007) High-level production of beta-carotene in Saccharomyces cerevisiae by successive transformation with carotenogenic genes from Xanthophyllomyces dendrorhous. Appl Environ Microbiol 73:4342–4350
Xie W, Liu M, Lv X, Lu W, Gu J, Yu H (2014) Construction of a controllable β-carotene biosynthetic pathway by decentralized assembly strategy in Saccharomyces cerevisiae. Biotechnol Bioeng 111:125–133
Xie W, Lv X, Ye L, Zhou P, Yu H (2015) Construction of lycopene-overproducing Saccharomyces cerevisiae by combining directed evolution and metabolic engineering. Metab Eng 30:69–78
Yamano S, Ishii T, Nakagawa M, Ikenaga H, Misawa N (1994) Metabolic engineering for production of β-carotene and lycopene in Saccharomyces cerevisiae. Biosci Biotechnol Biochem 58:1112–1114
Yoon SH, Kim JE, Lee SH, Park HM, Choi MS, Kim JY, Lee SH, Shin YC, Keasling JD, Kim SW (2007) Engineering the lycopene synthetic pathway in E. coli by comparison of the carotenoid genes of Pantoea agglomerans and Pantoea ananatis. Appl Microbiol Biotechnol 74:131–139
Yoon S, Lee S, Amitabha D, Ryu H, Jang H, Kim J, Oh D, Keasling JD, Kim S (2009) Combinatorial expression of bacterial whole mevalonate pathway for the production of β-carotene in E. coli. J Biotechnol 140:218–226
Zhou P, Ye L, Xie W, Lv X, Yu H (2015) Highly efficient biosynthesis of astaxanthin in Saccharomyces cerevisiae by integration and tuning of algal crtZ and bkt. Appl Microbiol Biotechnol 99:8419–8428
Zhou P, Xie W, Li A, Wang F, Yao Z, Bian Q, Zhu Y, Yu H, Ye L (2017) Alleviation of metabolic bottleneck by combinatorial engineering enhanced astaxanthin synthesis in Saccharomyces cerevisiae. Enzyme Microb Technol 100:28–36
Zhu C, Yamamura S, Nishihara M, Koiwa H, Sandmann G (2003) cDNAs for the synthesis of cyclic carotenoids in petals of Gentiana lutea and their regulation during flower development. Biochim Biophys Acta 1625:305–308
Acknowledgements
The authors thank to Akiko Kubo, Dr. Yoshinobu Terada, Dr. Kohji Ohdan (Ezaki Glico Co., Ltd.), Dr. Maiko Furubayashi, Dr. Katsuro Yaoi [National Institute of Advanced Industrial Science and Technology (AIST)], Dr. Masahiro Murata, and Prof. Michihiro Araki (Graduate School of Medicine, Kyoto University), for their collaborations with us. We are also grateful to Prof. Kazutoshi Shindo (Japan Women’s University) and Dr. Takashi Maoka (Research Institute for Production Development) for information on carotenoid distribution in plants.
Funding
This study was funded by “Smart Cell Project” organized by the New Energy and Industrial Technology Development Organization (NEDO) (16100920-0).
Author information
Authors and Affiliations
Contributions
MT and TS wrote the manuscript partially, and NM wrote the manuscript mainly. TS conducted experiments for the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Takemura, M., Sahara, T. & Misawa, N. Violaxanthin: natural function and occurrence, biosynthesis, and heterologous production. Appl Microbiol Biotechnol 105, 6133–6142 (2021). https://doi.org/10.1007/s00253-021-11452-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11452-2