Abstract
Background
Microbial production of lycopene, a commercially and medically important compound, has received increasing concern in recent years. Saccharomyces cerevisiae is regarded as a safer host for lycopene production than Escherichia coli. However, to date, the lycopene yield (mg/g DCW) in S. cerevisiae was lower than that in E. coli and did not facilitate downstream extraction process, which might be attributed to the incompatibility between host cell and heterologous pathway. Therefore, to achieve lycopene overproduction in S. cerevisiae, both host cell and heterologous pathway should be delicately engineered.
Results
In this study, lycopene biosynthesis pathway was constructed by integration of CrtE, CrtB and CrtI in S. cerevisiae CEN.PK2. When YPL062W, a distant genetic locus, was deleted, little acetate was accumulated and approximately 100 % increase in cytosolic acetyl-CoA pool was achieved relative to that in parental strain. Through screening CrtE, CrtB and CrtI from diverse species, an optimal carotenogenic enzyme combination was obtained, and CrtI from Blakeslea trispora (BtCrtI) was found to have excellent performance on lycopene production as well as lycopene proportion in carotenoid. Then, the expression level of BtCrtI was fine-tuned and the effect of cell mating types was also evaluated. Finally, potential distant genetic targets (YJL064W, ROX1, and DOS2) were deleted and a stress-responsive transcription factor INO2 was also up-regulated. Through the above modifications between host cell and carotenogenic pathway, lycopene yield was increased by approximately 22-fold (from 2.43 to 54.63 mg/g DCW). Eventually, in fed-batch fermentation, lycopene production reached 55.56 mg/g DCW, which is the highest reported yield in yeasts.
Conclusions
Saccharomyces cerevisiae was engineered to produce lycopene in this study. Through combining host engineering (distant genetic loci and cell mating types) with pathway engineering (enzyme screening and gene fine-tuning), lycopene yield was stepwise improved by 22-fold as compared to the starting strain. The highest lycopene yield (55.56 mg/g DCW) in yeasts was achieved in 5-L bioreactors. This study provides a good reference of combinatorial engineering of host cell and heterologous pathway for microbial overproduction of pharmaceutical and chemical products.
Similar content being viewed by others
Background
Artificial biosynthetic pathway and host cell are two fundamental elements for microbe-based heterologous biosynthesis of natural products. On one hand, potential metabolic and regulatory issues from host cell play an important role in pathway productivity [1–4]. One-hundred distant genetic loci that are not directly involved in target pathway were identified to influence carotenoid production significantly in Saccharomyces cerevisiae [5]. On the other hand, balanced metabolic flux between modules in target pathway is another important issue to improve pathway performance. Through multivariate-modular optimization of taxadiene metabolic pathway, a 15,000-fold increase in taxadiene titer was observed in Escherichia coli [6]. A “push–pull-block” pathway manipulation strategy significantly enhanced terpenoids production in yeasts [7, 8]. Thus, optimal pathway output can be achieved by means of delicate engineering of both target pathway and host cell [9]. It was reported that bisabolene production in S. cerevisiae was increased by 20 times through deleting multiple distant genes related to intracellular mevalonate level and manipulating the expression level of three genes involved in mevalonate (MVA) pathway [10]. Swidah et al. [11] reported that through the combinatorial effects of deletion of ADH1 to restore redox imbalance, expression of a butanol resistant allele GCD1, and manipulation of acetyl-CoA formation module, butanol production in S. cerevisiae was increased by more than 30 times. In a word, combinatorial engineering host cell with heterologous pathway offers a promising alternative to achieve better metabolic flux balance and higher output of heterologous pathway.
Lycopene has long been used as functional food, nutraceutical, pharmaceutical and cosmetic due to its anti-oxidative and anti-cancer activities [12, 13]. Compared to chemical synthesis and extraction from tomatoes, microbial production of lycopene is more economical and sustainable. In recent years, lycopene production was successfully realized in Blakeslea trispora, E. coli and yeasts. However, regarding to food safety issues, it is controversial to use B. trispora or E. coli for lycopene synthesis, since E. coli would release endotoxin [14] and B. trispora requires the addition of cyclase inhibitors [15]. Saccharomyces cerevisiae is generally recognized as safe (GRAS), robust and preferred organism for industrial use. To date, lycopene yield in S. cerevisiae was increased to 24.41 mg/g DCW with elaborate efforts in directed evolution and copy number variation of Crt genes from Xanthophyllomyces dendrorhous [16]. However, the lycopene yield was still much lower than that in E. coli [17, 18], which did not facilitate downstream extraction process. It was speculated that such low yield might be attributed to the incompatibility between S. cerevisiae and the heterologous pathway. Therefore, combinatorial engineering S. cerevisiae with a heterologous pathway may offer an effective solution to enhance lycopene yield.
In this study, heterologous carotenogenic pathway and its recruited host S. cerevisiae were combinatorially engineered (Fig. 1c). Acetyl-CoA formation was enhanced by the deletion of YPL062W. A novel and optimal combination of geranylgeranyl diphosphate synthase (CrtE, or GGPPS), phytoene synthase (CrtB) and phytoene desaturase (CrtI) was generated through related enzyme screening from diverse species. The expression level of CrtI, which has significant impact on lycopene production as well as lycopene proportion in carotenoid, was fine-tuned by varying promoter strength and copy number. The influences of different cell mating types and potential distant genetic loci were also evaluated. Using this combinatorial engineering strategy, we achieved approximately 22-fold improvement (up to 54.63 mg/g DCW) in lycopene yield, which provides a good reference to increase the compatibility between heterologous pathway and host cell for microbial production of valuable molecules.
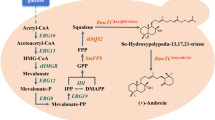
Schematic representation of the engineering strategies for enhanced lycopene production in S. cerevisiae. a The engineered lycopene biosynthetic pathway in S. cerevisiae. Genetic modifications are noted by thick arrows. Red arrows indicate the heterologous lycopene biosynthetic pathway consisting of CrtE, CrtB and CrtI, which starts either from FPP (solid arrow) or directly from IPP and DMAPP (dashed arrow). The enzyme overexpressed in the described lycopene-producing strain is highlighted in blue. b Construction of plasmids and integration modules. c The rationale of experiment design: combinatorial engineering of host cell and heterologous pathway
Methods
Materials
All oligonucleotides were purchased from Invitrogen. Q5 DNA polymerase and PmeI restriction endonuclease were purchased from New England Biolabs (MA, USA). DNA purification and plasmids isolation kits were purchased from Tiangen (Beijing, China). CloneJET PCR Cloning Kit was purchased from Fermentas (MD, USA). DNA sequencing was conducted by Genewiz (Beijing, China). Standards of lycopene and phytoene were purchased from Sigma (Sigma-Aldrich, MO, USA). Standards of phytofluene, ζ-carotene and neurosporene were purchased from Express (Beijing, China).
Strains and culture conditions
Escherichia coli DH5α was used for routine cloning procedures, and was cultivated at 37 °C in Luria–Bertani (LB) medium containing 100 μg/mL ampicillin for selection. All the yeast strains engineered in this study are based on homologous haploid S. cerevisiae strains, CEN.PK2-1C (MATa) or CEN.PK2-1D (MATα). Engineered yeast strains were selected on synthetic complete (SC) medium (0.67 % yeast nitrogen base without amino acids, 2 % glucose, and appropriate amino acid drop-out mix), or YPD medium (2 % peptone, 1 % yeast extract, and 2 % glucose) with 200 μg/mL geneticin, 300 μg/mL hygromycin B or 50 μg/mL bleomycin when needed. YPDG medium, consisting of 2 % glucose (unless otherwise indicated), 2 % peptone, 1 % yeast extract and 1 % D-(+)-galactose, was used for shake-flask fermentations.
For shake-flask fermentation, yeast glycerol-stock was inoculated into a tube containing 2 mL YPD medium for overnight growth, then all the preculture was inoculated into a 250 mL shake-flask containing 25 mL YPD. After growing to the mid-log phase, the seed was transferred to 50 mL fresh YPDG medium at an initial OD600 of 0.5 and grown at 30 °C for 48 h.
Construction of plasmids and strains
Saccharomyces cerevisiae strains and plasmids used in this study are summarized in Table 1. All oligonucleotides used for construction of the above plasmids and strains are listed in Additional file 1: Table S1. Construction procedures of plasmids and integration modules are shown in Fig. 1b. All heterologous genes used for lycopene biosynthesis were codon-optimized and synthesized by Genewiz (Beijing, China) for expression in S. cerevisiae. Endogenous truncated 3-hydroxy-3-methylglutaryl coenzyme A reductase (tHMG1) [19], promoters, terminators and integration homologous arms (except TRP1 homologous arm) used in this study were amplified from the genomic DNA of S. cerevisiae CEN.PK2-1C. Auxotroph markers (LEU2, HIS3) and TRP1 homologous arm were amplified from the genomic DNA of S. cerevisiae S288C. Antibiotic markers (KanMX, HphMX and BleMX) were amplified from plasmid pKan, pHph and pBle (owned by our laboratory), respectively. Overlap extension PCR (OE-PCR) was used to assemble the above parts into modules according to Additional file 1: Figure S1. The resulting modules were purified and cloned into pJET1.2/blunt vector following the protocol of CloneJET PCR Cloning Kit (Fermentas, MD, USA), obtaining plasmid pCY series (Table 1). Finally, all the integration modules were digested from plasmids with PmeI, then purified and transformed into yeast for genomic integration using the LiAc/SS carrier DNA/PEG method [20]. For gene deletions, one-step integration of PCR-amplified deletion cassettes was adopted [21]. Gene deletions and genomic integrations were verified by diagnostic PCR.
Assay of extracellular glucose, ethanol, acetate and glycerol
The concentrations of residual glucose, ethanol, acetate and glycerol in the medium were determined by HPLC (Waters Corp., USA) with a refractive index detector. Aminex HPX-87H column (BioRad, CA) was used for separation at the column temperature of 65 °C. 5 mM H2SO4 was used as eluent with a flow rate of 0.6 mL/min.
Assay of acetyl-CoA
Cells were sampled during the course of lycopene shake-flask fermentation for acetyl-CoA assay. Acetyl-CoA was extracted as previously described [22] and analyzed by the acetyl-CoenzymeA Assay Kit (Sigma-Aldrich). The acetyl-CoA concentration was normalized by dry cell weight.
Assay of promoter strength
Fluorescence intensity of red fluorescence protein (RFP) was used to characterize the strengths of GAL3, GAL7 and GAL10 promoters as previously described [23]. The strain without promoter fused with RFP (SyBE_Sc14C40) was used as the negative control. Culturing procedures of all the test strains (SyBE_Sc14C40–SyBE_Sc14C43; Table 1) were the same as lycopene fermentation in shake-flasks. Every 6 h of cultivation, cells were harvested, washed and diluted with phosphate-buffered saline (PBS) into an OD600 of 0.3–0.4 for fluorescence assay. RFP fluorescence intensity was detected by SpectraMax M2 microplate reader with excitation and emission wavelengths at 587 and 611 nm, respectively. Promoter strength was determined as the ratio of the fluorescence to OD600 for each strain.
Extraction and analysis of carotenoid
Extraction of carotenoid was as described by Xie et al. [24] with some modifications. Briefly, cells harvested from cultures were washed, resuspended in boiling 3 N HCl for 2 min, and cooled in an ice-bath for 3 min. Then, cells debris were washed twice with water, resuspended in acetone containing 1 % BHT (w/v), vortexed with glass beads (425–600 µm, Sigma) until colorless, and followed by centrifugation. The acetone phase containing the extracted carotenoid was filtered for HPLC analysis. A HPLC system (Waters e2695) equipped with a BDS Hypersil C18 column (4.6 × 150 mm, 5 µm) and a UV/VIS detector (Waters 2489) was used to analyze the produced carotenoid. The signals of phytoene, phytofluene, ζ-carotene, neurosporene and lycopene were detected at 287, 349, 401, 440 and 471 nm, respectively [25]. The mobile phase consisted of methanol–acetonitrile-dichloromethane (21:21:8 v/v) with a flow rate of 1 mL/min at 30 °C [26]. Total carotenoid was calculated as the sum of the above carotenoids.
Microscopy
Microscopic analysis was used to investigate lycopene formation of strain SyBE_Sc14C45 during shake-flask fermentation with YPDG medium. SyBE_Sc14C45 cultivated in YPD medium (without galactose) was used as control. After 36 h of cultivation, cells were harvested, washed and diluted with sterile water into an OD600 of 5.0. Images were taken with an Olympus CX41 (Olympus, Tokyo, Japan).
Fed-batch fermentation
Strain SyBE_Sc14D14 was selected for fed-batch fermentation. Seed cultures were prepared by inoculating 250 µL of glycerol-stock into a 250 mL shake-flask containing 25 mL YPD and culturing at 30 °C for 16 h to an OD600 of 6–7, and then 15 mL of precultures were inoculated into a 2 L shake-flask containing 400 mL YPD and subcultured for an additional 8 h at 30 °C to an OD600 of 5–6. Seed cultures were transferred into a 5 L bioreactor (BaiLun, China) containing 2 L YPD batch medium at a 10 % (v/v) inoculum. Fermentation was carried out at 30 °C with an air flow rate of 1.5 vvm. The dissolved oxygen was kept at 30 % by adjusting the agitation speed from 400 to 700 rpm and pH was controlled at 6.0 by automatic addition of 6 M sodium hydroxide.
According to the employed galactose-inducible system for lycopene biosynthesis, fed-batch fermentation was divided into two stages: cell growth stage and lycopene production stage. During the first stage to achieve maximal cell growth, concentrated glucose solution (500 g/L) was fed periodically into bioreactors to keep the glucose concentration under 2 g/L. In the meanwhile, 100 mL of the concentrated yeast extract solution (400 g/L) was fed periodically into the bioreactor every 10 h. Once cell growth entered stationary phase, glucose and yeast extract feedings were ceased, and 10 g/L of D-(+)-galactose was added to induce lycopene biosynthesis. After the depletion of the residual glucose, cells began to consume ethanol converted by glucose consumption. Ethanol concentration was controlled below 5 g/L by adjusting 100 % ethanol feeding rate until harvest.
Results and discussion
Construction of inducible lycopene biosynthesis pathway
To avoid the potential toxicity of lycopene, genes responsible for carotenoid synthesis were placed under the control of galactose-regulated GAL promoters. Δgal1 Δgal7 Δgal10 and Δgal80 were two routine strategies to employ GAL promoters, since Δgal1 Δgal7 Δgal10 eliminates galactose utilization and Δgal80 does not require galactose for induction [27]. Here, S. cerevisiae SyBE_Sc14C01 (Δgal80) and SyBE_Sc14C02 (Δgal1 Δgal7 Δgal10) were chosen as the host cells for lycopene production. The carotenogenic pathway was constructed by genomic integration of CrtE, CrtB, CrtI and tHMG1 in the respective hosts (Fig. 1a). As a result, strain SyBE_Sc14C07 (Δgal1 Δgal7 Δgal10) with CrtE and CrtB from Pantoea agglomerans and CrtI from Blakeslea trispora produced 78.8 % higher lycopene yield (4.31 mg/g DCW) than strain SyBE_Sc14C06 (Δgal80) harboring the same Crt genes (2.43 mg/g DCW) after 48 h of shake-flask culture in YPDG medium (Additional file 1: Figure S2). Thus, strain SyBE_Sc14C02 (Δgal1 Δgal7 Δgal10) was selected as the host cell for the further study.
Enhancement of acetyl-CoA pool by the deletion of YPL062 W
Δypl062w (Additional file 1: Table S2) was previously reported to enhance carotenoid production by increasing the intracellular mevalonate level [10], but the mechanism was not clear. In order to testify whether Δypl062w is benefit to lycopene production, YPL062W was deleted in SyBE_Sc14C07, generating strain SyBE_Sc14C23. As a result, Δypl062w increased lycopene yield by more than 1.5-fold when cultured in YPDG medium with 2 % glucose (Fig. 2a), which is consistent with previous work [10]. Compared to strain SyBE_Sc14C07 with 0.51 g/L extracellular acetate accumulation, no acetate accumulation was observed in strain SyBE_Sc14C23 (Fig. 2c). Furthermore, SyBE_Sc14C07 and SyBE_Sc14C23 were cultivated in YPDG media with higher (4 %) glucose. As shown in Fig. 2b and d, 10.84 mg/g DCW of lycopene together with very little amount of acetate was detected in SyBE_Sc14C23, whereas only 70 µg/g DCW of lycopene and up to 5.04 g/L of acetate were obtained in SyBE_Sc14C07. It was also observed that cell growth of strain without Δypl062w was abolished when acetate accumulated up to 1.0 g/L (Fig. 2d), which is in accordance with Thomas et al. [28]. Moreover, when SyBE_Sc14C07 was cultured in YPDG medium with 2 % glucose, different concentrations (0, 0.5, 1.0, 1.5 g/L) of acetic acid were added to the media at the time of 7 h when glucose was exhausted (indicated as arrow in Additional file 1: Figure S3). Cell growth was also abolished when the added acetic acid concentration exceeded 1.0 g/L (Additional file 1: Figure S3A). Lycopene yield was dropped by 98.4 % (from 4.31 mg/g DCW to 67 µg/g DCW) when additional 0.5 g/L acetic acid was added (Additional file 1: Figure S3B). No lycopene production was detected when 1.0 g/L acetic acid was added (Additional file 1: Figure S3B), suggesting acetate accumulation would be harmful to lycopene biosynthesis. Thus, Δypl062w acted as an important role in S. cerevisiae to reduce acetate accumulation.
The effect of Δypl062w on lycopene production. S. cerevisiae SyBE_Sc14C07 and SyBE_Sc14C23 were cultivated in YPDG media containing different concentrations of glucose (2 %, left side; 4 %, right side), respectively, in shake-flasks for analysis of lycopene production (a, b), acetate accumulation (c, d) and cytosolic acetyl-CoA level (e, f). The error bars represent standard deviation calculated from triplicate experiments
As is known, acetate is the direct precursor for cytosolic acetyl-CoA. Therefore, we assumed that Δypl062w would enhance cytosolic acetyl-CoA pool from acetate. As expected, the cytosolic acetyl-CoA concentrations in SyBE_Sc14C23 were increased by approximately 100 % than those in SyBE_Sc14C07 at early times (Fig. 2e, f). When lycopene was rapidly accumulated, SyBE_Sc14C23 demonstrated the same cytosolic acetyl-CoA level but higher lycopene production as compared to SyBE_Sc14C07 (Fig. 2a, b, e, f). This result suggested the expansion on lycopene production might be derived from the increase in acetyl-CoA supplement, since the intracellular mevalonate level of Δypl062w strain was significantly increased during terpenoid production [10]. Therefore, Δypl062w enhanced “trapping” the carbon from acetate accumulation toward acetyl-CoA, which consequently improved MVA pathway flux and further lycopene production. Our findings made us a better understanding of the effect of Δypl062w on MVA pathway.
Optimal combination of CrtE, CrtB and CrtI by screening enzymes from diverse sources
It is known that enzymes from different organisms often vary in catalytic activities [29, 30]. Besides, host cell compatibility may also affect the optimal performance of heterologous enzymes [16, 31]. Thus, screening enzymes from diverse sources offers an effective strategy to increase the productivity of heterologous pathway in specific host [29, 32]. To date, most of the carotenogenic genes employed in heterologous biosynthesis were derived from Pantoea, Paracoccus or Xanthophyllomyces species [33, 34]. However, in S. cerevisiae, the currently reported Crt genes for high-level carotenoid production were only from X. dendrorhous [16, 24, 35]. In this study, we aimed to rebuild a carotenogenic pathway with high productivity in S. cerevisiae by screening enzymes (CrtE, CrtB, and CrtI) from some other species except X. dendrorhous. Five CrtEs originated from P. agglomerans (PaCrtE), Sulfolobus acidocaldarius (SaCrtE), Archaeoglobus fulgidus (AfCrtE), B. trispora (BtCrtE) and Taxus x media (TmCrtE), two CrtBs from P. agglomerans (PaCrtB) and Paracoccus sp. (formerly Agrobacterium aurantiacum) (AaCrtB), and three CrtIs from P. agglomerans (PaCrtI), Paracoccus sp. (AaCrtI) and B. trispora (BtCrtI) were selected for carotenoid biosynthesis. As illustrated in Fig. 3, thirty lycopene-producing strains were constructed and their production was investigated. Consequently, the lycopene yield in strain SyBE_Sc14C35 harboring the best enzyme combination (TmCrtE, PaCrtB and BtCrtI) was increased by 7.5-fold, up to 36.75 mg/g DCW, and the lycopene proportion in carotenoid was 64.11 % (Fig. 3). This strain was used as the candidate for the further optimization.
Combinatorial optimization of CrtE, CrtB and CrtI from diverse species. Thirty lycopene-producing strains were constructed by screening enzymes from various sources and tested for lycopene production. Pa, Pantoea agglomerans; Sa, Sulfolobus acidocaldarius; Af, Archaeoglobus fulgidus; Bt, Blakeslea trispora; Tm, Taxus x media; Aa, Paracoccus sp. (formerly Agrobacterium aurantiacum). The error bars represent standard deviation calculated from triplicate experiments
In general, CrtE, one of the rate-limiting enzymes in carotenoid pathway [36], was found to be crucial to the production yield of overall carotenoid. As shown in Fig. 3, strains harboring AfCrtE, BtCrtE or TmCrtE showed much higher yield of total carotenoid than that harboring PaCrtE or SaCrtE. TmCrtE was reported to possess a larger pocket and higher affinity for farnesyl diphosphate (FPP) binding than other GGPPSs [30], thus it was reasonable that strain with TmCrtE achieved high carotenoid yield. CrtE from B. trispora [37] was firstly well expressed in S. cerevisiae and achieved quite high yield of overall carotenoid, demonstrating that BtCrtE would be a promising GGPPS candidate for further study. AfCrtE can directly utilize dimethylallyl pyrophosphate (DMAPP)/isopentenyl pyrophosphate (IPP) to synthesize geranylgeranyl diphosphate (GGPP) and thus avoid competing FPP for sterols biosynthesis [38], which might explain the improved carotenoid production catalyzed by AfCrtE. In addition to AfCrtE, SaCrtE was also a bifunctional FPP/GGPP synthase [39] and has been demonstrated to increase diterpenoids production [40, 41]. But it was difficult to interpret the low carotenoid yield by SaCrtE according to our current data, which might be ascribed to the insufficient expression of SaCrtE or its incompatibility with CrtB and CrtI.
Phytoene, synthesized by CrtB from GGPP, is the first intermediate of the carotenoid pathway. Then lycopene was generated by CrtI through four successive dehydrogenation steps from phytoene (Fig. 1a). As illustrated in Fig. 3, rather than lycopene, phytoene was one of the major components of the total carotenoid in most of our engineered strains, indicating that CrtI-catalyzed conversion from phytoene to lycopene was another rate-limiting step, which is consistent with previous reports [16, 35]. To be noted, all the strains harboring BtCrtI showed much better performance than strains harboring PaCrtI or AaCrtI, irrespective of lycopene yield or proportion in carotenoid (Fig. 3). BtCrtI, an enzyme from eukaryotic organsims, was firstly well expressed in S. cerevisiae and found to be more suitable for high-level conversion from phytoene to lycopene in S. cerevisise according to our results. The molecular mechanism for its high efficiency in conversion from phytoene to lycopene is an interesting topic in the field in future.
Fine-tuning of BtCrtI and selection of homologous haploid yeast hosts
Despite the improved lycopene yield in SyBE_Sc14C35, approximately 26 % of the total carotenoid was phytoene, which indicated that the phytoene conversion directed by BtCrtI was not efficient enough. In order to achieve higher lycopene proportion, the expression level of BtCrtI needs to be fine-tuned. Here, BtCrtI was fine-tuned by adjusting different promoters and integration copy numbers. The strengths of GAL promoters used in this step were characterized in strain SyBE_Sc14C10 (CEN.PK2-1C, Δgal1 Δgal7 Δgal10, Δypl062w), and P GAL3 was found to be the weakest one (Additional file 1: Figure S4). When one additional copy of BtCrtI under the control of P GAL3 or P GAL7 was integrated in strain SyBE_Sc14C35, the lycopene proportion in the resulting strain SyBE_Sc14C44 or SyBE_Sc14C45 was increased by 17.9 % (from 64.11 to 75.58 %) or 35.2 % (up to 86.68 %), respectively (Fig. 4). Moreover, a slight decrease in lycopene yield was observed after another additional copy of P GAL7 -BtCrtI integrated in SyBE_Sc14C45 (Fig. 4), which is similar to recent report [16]. To be noted, although the lycopene yield was modestly increased, the total carotenoid yield was decreased obviously after fine-tuning steps for higher lycopene proportion (Fig. 4). As shown in Additional file 1: Figure S5, most of the lycopene was accumulated in cell membrane, which is consistent with early reports [42, 43]. Rapid lycopene accumulation in cell membrane would lead to membrane stress or cell toxicity, which might explain the significant decrement in the content of total carotenoid after fine-tuning. Therefore, increasing lycopene tolerance in S. cerevisiae would be an effective direction to improve lycopene yield.
Carotenoid production by fine-tuning of BtCrtI and selecting homologous haploid strains. BtCrtI expression was fine-tuned by adjusting copy number and promoter strength, and haploid cells with different mating types (a, α) were compared as well for carotenoid production. The error bars represent standard deviation calculated from triplicate experiments
Haploid cell type was reported to have significant influence on heterologous terpenoid production [44]. Jackson et al. [45] found that MATa strain was more suitable to produce epi-cedrol than MATα strain, since MATa strain might synthesize more FPP for prenylation of the mating pheromone [46]. However, cell mating types did not have significant effect on linalool production [47]. Here, lycopene production in CEN.PK2-1C (MATa) and CEN.PK2-1D (MATα) with the same genetic modifications was evaluated, respectively. The strains (SyBE_Sc14D05–SyBE_Sc14D08) derived from CEN.PK2-1D (MATα) achieved 12–15 % higher lycopene yield than strains (SyBE_Sc14C35, SyBE_Sc14C44–SyBE_Sc14C46) from CEN.PK2-1C (MATa) (Fig. 4), suggesting that MATα strain was preferred in the case of lycopene production. Finally, the strain SyBE_Sc14D07 (MATα) achieved lycopene yield of 46.26 mg/g DCW with a proportion of 82.36 % (Fig. 4).
Effects of distant genetic loci on lycopene production
As the engineered metabolic pathways were highly inter-connected with the rest of cellular metabolism and tightly regulated [48], distantly located genetic loci in host cell could also have potential interactions with target pathway. As Δrox1, Δdos2 and Δyjl064w (Additional file 1: Table S2) were proved to greatly benefit carotenoid production [5, 10], these three distant genetic loci were knocked out individually in strain SyBE_Sc14D07. As shown in Fig. 5, both Δrox1 and Δdos2 conferred a modest increase (8.7 and 5.7 %, respectively) in lycopene production as expected, while Δyjl064w led to 18.2 % decreased lycopene yield as compared to SyBE_Sc14D07. Moreover, the combination of Δrox1 and Δdos2 did not show a synergistic effect on lycopene production (Fig. 5), which is inconsistent with the results obtained by Trikka et al. [5]. These inconsistencies might be attributed to that the impact of perturbations in one strain may not be directly applied to another strain with a modified genetic background [1]. Consequently, a relatively higher lycopene yield of 50.28 mg/g DCW was obtained in SyBE_Sc14D11 with Δrox1.
The effects of distant genetic loci on lycopene production. Three gene-deletion targets (ROX1, DOS2, and YJL064W) and one overexpression target (INO2) were investigated in S. cerevisiae SyBE_Sc14D07 for lycopene production. The error bars represent standard deviation calculated from triplicate experiments
As shown in Additional file 1: Figure S5, lycopene tended to accumulate in cell membrane and thus cause cell toxicity. Here, INO2 (Additional file 1: Table S2), an endogenous transcription factor related to cellular stress response [49, 50], was overexpressed in SyBE_Sc14D11. As a result, a lycopene yield of 54.63 mg/g DCW, the highest yield reported, was obtained in the resulting strain SyBE_Sc14D14 in shake-flask cultivation (Fig. 5). INO2 was reported to alleviate alkanes (C9–C11) toxicity by regulating genes associated with efflux pumps, stress response, lipid metabolism and ergosterol biosynthesis in S. cerevisiae [51]. High-level expression of INO2 was proved to up-regulate phospholipid and sterol biosynthesis [52]. Therefore, increasing lycopene tolerance through modifying membrane components (i.e. lipid, and ergosterol) may be the main reason for the improvement of lycopene yield via INO2. Therefore, INO2 was identified as a novel target for lycopene production in S. cerevisiae. Strain SyBE_Sc14D14 was chosen for fed-batch fermentation.
Lycopene overproduction in fed-batch fermentation
To evaluate the production performance of the engineered strain SyBE_Sc14D14, fed-batch fermentation was performed at a 2 L scale using YPD as the medium (Fig. 6). Based on carbon restriction strategy, trace amount of acetate was observed during the whole process (Additional file 1: Figure S6). Eventually, a total carotenoid titer of 1.81 g/L (60.94 mg/g DCW), consisting of 3.99 % of phytoene, 4.87 % of neurosporene and 91.14 % of lycopene, was obtained after 120 h of cultivation (Fig. 6). Lycopene yield of 55.56 mg/g DCW achieved in our work was the highest yield in yeast strains to date. However, the lycopene titer (1.65 g/L) was just similar to previous work by Xie et al. [16]. This is due to the relative low cell density, since the highest OD600 only reached 106 throughout the fermentation. Additionally, excessive accumulations of ethanol and glycerol were also observed during cell growth phase (Fig. 6a; Additional file 1: Figure S6), which competed carbon flow from biomass synthesis and implied redox imbalance of our engineered strain [53]. In future, to limit glucose below 0.5 g/L in growth phase will greatly increase cell density as well as reduce by-product (ethanol or glycerol) accumulation. Moreover, intracellular and extracellular metabolomics analysis will be an efficient way to find some biomarkers for batch media or feeding solution optimization. Off-gas analysis will also be promising for more precise process control. As recent studies in media optimization have demonstrated great potential in lycopene overproduction [16, 54], we believe that lycopene production by our engineered strain would be further improved by continuous efforts in both metabolic engineering and fermentation optimization.
Lycopene production in fed-batch fermentation. a Profile of glucose, ethanol, cell density and lycopene accumulation of strain SyBE_Sc14D14 during fed-batch fermentation. b Percentage ratios of the produced carotenoid composition at 120 h. The red liquid in the bottles was the lycopene fermentation broth. The error bars represent standard deviation calculated from duplicate experiments
Conclusions
In this work, lycopene overproduction was realized by combinatorial engineering of S. cerevisiae and lycopene biosynthesis pathway. Extracellular acetate accumulation was reduced and cytosolic acetyl-CoA pool was enhanced through the deletion of YPL062W. A novel and optimal combination of CrtE, CrtB and CrtI was obtained by screening enzymes from diverse sources. It was also found that CrtI from B. trispora had significant influence on lycopene yield as well as proportion in carotenoid. The proportion of lycopene was significantly increased via fine-tuning of CrtI. Then the effects of cell mating types, several potential distant targets (YJL064 W, ROX1, and DOS2), and INO2, a stress-related transcription factor, were also investigated. Lycopene yield was stepwise improved by approximately 22-fold as compared to the starting strain. The highest reported lycopene yield (55.56 mg/g DCW) and titer (1.65 g/L) were achieved in 5-L bioreactors, providing a good example for microbial overproduction of pharmaceutical and chemical products through combinatorial engineering of host cell and heterologous pathway.
Abbreviations
- MVA:
-
mevalonate
- HMG-CoA:
-
3-hydroxy-3-methylglutaryl coenzyme A
- IPP:
-
isopentenyl pyrophosphate
- DMAPP:
-
dimethylallyl pyrophosphate
- FPP:
-
farnesyl diphosphate
- GGPP:
-
geranylgeranyl diphosphate
- tHMG1:
-
truncated 3-hydroxy-3-methylglutaryl coenzyme A reductase
- CrtE:
-
geranylgeranyl diphosphate synthase (GGPPS)
- CrtB:
-
phytoene synthase
- CrtI:
-
phytoene desaturase
- Aa:
-
Paracoccus sp. (formerly Agrobacterium aurantiacum)
- Pa:
-
Pantoea agglomerans
- Sa:
-
Sulfolobus acidocaldarius
- Af:
-
Archaeoglobus fulgidus
- Bt:
-
Blakeslea trispora
- Tm:
-
Taxus x media
References
Alper H, Jin YS, Moxley JF, Stephanopoulos G. Identifying gene targets for the metabolic engineering of lycopene biosynthesis in Escherichia coli. Metab Eng. 2005;7(3):155–64.
Alper H, Miyaoku K, Stephanopoulos G. Construction of lycopene-overproducing E. coli strains by combining systematic and combinatorial gene knockout targets. Nat Biotechnol. 2005;23(5):612–6.
Brochado AR, Patil KR. Model-guided identification of gene deletion targets for metabolic engineering in Saccharomyces cerevisiae. Methods Mol Biol. 2014;1152:281–94.
Jin YS, Stephanopoulos G. Multi-dimensional gene target search for improving lycopene biosynthesis in Escherichia coli. Metab Eng. 2007;9(4):337–47.
Trikka FA, Nikolaidis A, Athanasakoglou A, Andreadelli A, Ignea C, Kotta K, Argiriou A, Kampranis SC, Makris AM. Iterative carotenogenic screens identify combinations of yeast gene deletions that enhance sclareol production. Microb Cell Fact. 2015;14:60.
Ajikumar PK, Xiao WH, Tyo KE, Wang Y, Simeon F, Leonard E, Mucha O, Phon TH, Pfeifer B, Stephanopoulos G. Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science. 2010;330(6000):70–4.
Chen Y, Daviet L, Schalk M, Siewers V, Nielsen J. Establishing a platform cell factory through engineering of yeast acetyl-CoA metabolism. Metab Eng. 2013;15:48–54.
Lv X, Xie W, Lu W, Guo F, Gu J, Yu H, Ye L. Enhanced isoprene biosynthesis in Saccharomyces cerevisiae by engineering of the native acetyl-CoA and mevalonic acid pathways with a push-pull-restrain strategy. J Biotechnol. 2014;186:128–36.
Ma T, Deng Z, Liu T. Microbial production strategies and applications of lycopene and other terpenoids. World J Microbiol Biotechnol. 2016;32(1):15.
Özaydın B, Burd H, Lee TS, Keasling JD. Carotenoid-based phenotypic screen of the yeast deletion collection reveals new genes with roles in isoprenoid production. Metab Eng. 2013;15:174–83.
Swidah R, Wang H, Reid PJ, Ahmed HZ, Pisanelli AM, Persaud KC, Grant CM, Ashe MP. Butanol production in S. cerevisiae via a synthetic ABE pathway is enhanced by specific metabolic engineering and butanol resistance. Biotechnol Biofuels. 2015;8:97.
Sies H, Stahl W. Lycopene: antioxidant and biological effects and its bioavailability in the human. Proc Soc Exp Biol Med. 1998;218:121–4.
Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst. 1995;87(23):1767–76.
Ray BL, Raetz CR. The biosynthesis of gram-negative endotoxin. A novel kinase in Escherichia coli membranes that incorporates the 4′-phosphate of lipid A. J Biol Chem. 1987;262(3):1122–8.
Mantzouridou F, Tsimidou MZ. Lycopene formation in Blakeslea trispora. Chemical aspects of a bioprocess. Trends Food Sci Technol. 2008;19(7):363–71.
Xie W, Lv X, Ye L, Zhou P, Yu H. Construction of lycopene-overproducing Saccharomyces cerevisiae by combining directed evolution and metabolic engineering. Metab Eng. 2015;30:69–78.
Kim YS, Lee JH, Kim NH, Yeom SJ, Kim SW, Oh DK. Increase of lycopene production by supplementing auxiliary carbon sources in metabolically engineered Escherichia coli. Appl Microbiol Biotechnol. 2011;90(2):489–97.
Zhu F, Lu L, Fu S, Zhong X, Hu M, Deng Z, Liu T. Targeted engineering and scale up of lycopene overproduction in Escherichia coli. Process Biochem. 2015;50(3):341–6.
Donald KA, Hampton RY, Fritz IB. Effects of overproduction of the catalytic domain of 3-hydroxy-3-methylglutaryl coenzyme A reductase on squalene synthesis in Saccharomyces cerevisiae. Appl Environ Microbiol. 1997;63:3341–4.
Gietz RD, Schiestl RH. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat Protoc. 2007;2(1):31–4.
Longtine MS, McKenzie IIIA, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14(10):953–61.
Lian J, Si T, Nair NU, Zhao H. Design and construction of acetyl-CoA overproducing Saccharomyces cerevisiae strains. Metab Eng. 2014;24:139–49.
Cao YX, Xiao WH, Liu D, Zhang JL, Ding MZ, Yuan YJ. Biosynthesis of odd-chain fatty alcohols in Escherichia coli. Metab Eng. 2015;29:113–23.
Xie W, Liu M, Lv X, Lu W, Gu J, Yu H. Construction of a controllable beta-carotene biosynthetic pathway by decentralized assembly strategy in Saccharomyces cerevisiae. Biotechnol Bioeng. 2014;111(1):125–33.
Britton G. General carotenoid methods. Methods Enzymol. 1985;111(4):113–49.
Zhao J, Li Q, Sun T, Zhu X, Xu H, Tang J, Zhang X, Ma Y. Engineering central metabolic modules of Escherichia coli for improving beta-carotene production. Metab Eng. 2013;17:42–50.
Westfall PJ, Pitera DJ, Lenihan JR, Eng D, Woolard FX, Regentin R, Horning T, Tsuruta H, Melis DJ, Owens A, et al. Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. Proc Natl Acad Sci USA. 2012;109(3):E111–8.
Thomas KC, Hynes SH, Ingledew WM. Influence of medium buffering capacity on inhibition of Saccharomyces cerevisiae growth by acetic and lactic acids. Appl Environ Microbiol. 2002;68(4):1616–23.
Chang JJ, Thia C, Lin HY, Liu HL, Ho FJ, Wu JT, Shih MC, Li WH, Huang CC. Integrating an algal β-carotene hydroxylase gene into a designed carotenoid-biosynthesis pathway increases carotenoid production in yeast. Bioresour Technol. 2015;184:2–8.
Ding MZ, Yan HF, Li LF, Zhai F, Shang LQ, Yin Z, Yuan YJ. Biosynthesis of Taxadiene in Saccharomyces cerevisiae: selection of geranylgeranyl diphosphate synthase directed by a computer-aided docking strategy. PLoS ONE. 2014;9(10):e109348.
Greene JJ. Host cell compatibility in protein expression. Methods Mol Biol. 2004;267:3–14.
Sarria S, Wong B, Garcia Martin H, Keasling JD, Peralta-Yahya P. Microbial synthesis of pinene. ACS Synth Biol. 2014;3(7):466–75.
Misawa N, Shimada H. Metabolic engineering for the production of carotenoids in non-carotenogenic bacteria and yeasts. J Biotechnol. 1997;59(3):169–81.
Mata-Gomez LC, Montanez JC, Mendez-Zavala A, Aguilar CN. Biotechnological production of carotenoids by yeasts: an overview. Microb Cell Fact. 2014;13:12.
Verwaal R, Wang J, Meijnen JP, Visser H, Sandmann G, van den Berg JA, van Ooyen AJ. High-level production of beta-carotene in Saccharomyces cerevisiae by successive transformation with carotenogenic genes from Xanthophyllomyces dendrorhous. Appl Environ Microbiol. 2007;73(13):4342–50.
Yoon SH, Kim JE, Lee SH, Park HM, Choi MS, Kim JY, Lee SH, Shin YC, Keasling JD, Kim SW. Engineering the lycopene synthetic pathway in E. coli by comparison of the carotenoid genes of Pantoea agglomerans and Pantoea ananatis. Appl Microbiol Biotechnol. 2007;74(1):131–9.
Sun J, Sun XX, Tang PW, Yuan QP. Molecular cloning and functional expression of two key carotene synthetic genes derived from Blakeslea trispora into E. coli for increased β-carotene production. Biotechnol Lett. 2012;34(11):2077–82.
Wang CW, Oh MK, Liao JC. Engineered isoprenoid pathway enhances astaxanthin production in Escherichia coli. Biotechnol Bioeng. 1999;62(2):235–41.
Ohnuma SI, Suzuki M, Nishino T. Archaebacterial ether-linked lipid biosynthetic gene. Expression cloning, sequencing, and characterization of geranylgeranyl-diphosphate synthase. J Biol Chem. 1994;269(20):14792–7.
Dai Z, Liu Y, Huang L, Zhang X. Production of miltiradiene by metabolically engineered Saccharomyces cerevisiae. Biotechnol Bioeng. 2012;109(11):2845–53.
Engels B, Dahm P, Jennewein S. Metabolic engineering of taxadiene biosynthesis in yeast as a first step towards Taxol (Paclitaxel) production. Metab Eng. 2008;10(3–4):201–6.
Gruszecki WI, Strzalka K. Carotenoids as modulators of lipid membrane physical properties. Biochim Biophys Acta. 2005;1740(2):108–15.
Verwaal R, Jiang Y, Wang J, Daran JM, Sandmann G, van den Berg JA, van Ooyen AJ. Heterologous carotenoid production in Saccharomyces cerevisiae induces the pleiotropic drug resistance stress response. Yeast. 2010;27(12):983–98.
Ohto C, Muramatsu M, Obata S, Sakuradani E, Shimizu S. Overexpression of the gene encoding HMG-CoA reductase in Saccharomyces cerevisiae for production of prenyl alcohols. Appl Microbiol Biotechnol. 2009;82(5):837–45.
Jackson BE, Hart-Wells EA, Matsuda SP. Metabolic engineering to produce sesquiterpenes in yeast. Org Lett. 2003;5(10):1629–32.
Anderegg RJ, Betz R, Carr SA, Crabb JW, Duntze W. Structure of Saccharomyces cerevisiae mating hormone a-factor. Identification of S-farnesyl cysteine as a structural component. J Biol Chem. 1988;263(34):18236–40.
Rico J, Pardo E, Orejas M. Enhanced production of a plant monoterpene by overexpression of the 3-hydroxy-3-methylglutaryl coenzyme A reductase catalytic domain in Saccharomyces cerevisiae. Appl Environ Microbiol. 2010;76(19):6449–54.
Szappanos B, Kovács K, Szamecz B, Honti F, Costanzo M, Baryshnikova A, Gelius-Dietrich G, Lercher MJ, Jelasity M, Myers CL, et al. An integrated approach to characterize genetic interaction networks in yeast metabolism. Nat Genet. 2011;43(7):656–62.
Li BZ, Cheng JS, Ding MZ, Yuan YJ. Transcriptome analysis of differential responses of diploid and haploid yeast to ethanol stress. J Biotechnol. 2010;148(4):194–203.
Villa-Garcia MJ, Choi MS, Hinz FI, Gaspar ML, Jesch SA, Henry SA. Genome-wide screen for inositol auxotrophy in Saccharomyces cerevisiae implicates lipid metabolism in stress response signaling. Mol Genet Genomics. 2011;285(2):125–49.
Ling H, Chen B, Kang A, Lee JM, Chang MW. Transcriptome response to alkane biofuels in Saccharomyces cerevisiae: identification of efflux pumps involved in alkane tolerance. Biotechnol Biofuels. 2013;6(1):95.
Chumnanpuen P, Nookaew I, Nielsen J. Integrated analysis, transcriptome-lipidome, reveals the effects of INO-level (INO2 and INO4) on lipid metabolism in yeast. BMC Syst Biol. 2013;7(Suppl 3):S7.
Vemuri GN, Eiteman MA, McEwen JE, Olsson L, Nielsen J. Increasing NADH oxidation reduces overflow metabolism in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2007;104(7):2402–7.
Zhang C, Chen X, Zou R, Zhou K, Stephanopoulos G, Too HP. Combining genotype improvement and statistical media optimization for isoprenoid production in E. coli. PLoS ONE. 2013;8(10):e75164.
Authors’ contributions
YC, WX and YY conceived of the study and drafted the manuscript. YC carried out the molecular genetic studies. YC and WX carried out the fed-batch fermentation experiments. YW participated in design and coordination of the study and helped to draft the manuscript. HL and XL participated in HPLC analysis and data processing. YY supervised the whole research and revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors are grateful for the financial support from the Ministry of Science and Technology of China (“863” Program: 2012AA02A701) and the International S&T Cooperation Program of China (2015DFA00960).
Competing interests
The authors declare that they have no competing interests.
Availability of data and material
The material and data supporting their findings can be found in the main paper and the Additional file 1.
Funding
The Ministry of Science and Technology of China (“863”Program: 2012AA02A701) and the International S&T Cooperation Program of China (2015DFA00960) supported this work.
Author information
Authors and Affiliations
Corresponding author
Additional file
12934_2016_509_MOESM1_ESM.docx
Additional file 1. This file consists of two supplemental tables and seven supplemental figures. Table S1. Oligonucleotides used in this study. Table S2. Distant genetic loci involved in this study. Figure S1. Integration modules constructed in this study. Figure S2. Comparison of Δgal1 Δgal7 Δgal10 strain and Δgal80 strain on lycopene production. Figure S3. The effects of acetic acid addition on cell growth (A) and lycopene production (B) in strain SyBE_Sc14C07. Figure S4. Time course of promoter strengths. Figure S5. Microscopic images of lycopene-producing strain. Figure S6. Profile of glycerol and acetate accumulation. Figure S7. Sequences of codon-optimized genes.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chen, Y., Xiao, W., Wang, Y. et al. Lycopene overproduction in Saccharomyces cerevisiae through combining pathway engineering with host engineering. Microb Cell Fact 15, 113 (2016). https://doi.org/10.1186/s12934-016-0509-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-016-0509-4