Abstract
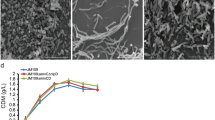
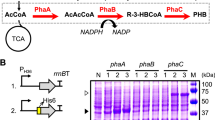
Polyhydroxyalkanoates (PHAs) can be produced by microorganisms from renewable resources and are regarded as promising bioplastics to replace petroleum-based plastics. A medium-chain-length PHAs (mcl-PHA)-producing strain Pseudomonas mendocina NK-01 was isolated previously by our lab and its whole-genome sequence is currently available. Morphology engineering of manipulating cell morphology–related genes has been applied for enhanced accumulation of the intracellular biopolymer short-chain-length PHAs (scl-PHA). However, it has not yet been reported to improve the yield of mcl-PHA by morphology engineering so far. In this work, several well-characterized cell morphology–related genes, including the cell fission ring (Z-ring) location genes minCD, peptidoglycan degradation gene nlpD, actin-like cytoskeleton protein gene mreB, Z-ring formation gene ftsZ, and FtsZ inhibitor gene sulA, were intensively investigated for their impacts on the cell morphology and mcl-PHA accumulation by gene knockout and overexpression in P. mendocina NKU, a upp knockout mutant of P. mendocina NK-01. For a minCD knockout mutant P. mendocina NKU-∆minCD, the average cell length was obviously increased and the mcl-PHA production was improved. However, the nlpD knockout mutant had a shorter cell length and lower mcl-PHA yield compared with P. mendocina NKU. Overexpression of mreB in P. mendocina NKU resulted in spherical cells. When ftsZ was overexpressed in P. mendocina NKU, the cell division was accelerated and the mcl-PHA titer was improved. Furthermore, mreB, ftsZ, or sulA was overexpressed in P. mendocina NKU-∆minCD. Consequently, the mcl-PHA titers were all increased compared with P. mendocina NKU-∆minCD carrying the empty vector. The multiple fission pattern was finally achieved in ftsZ-overexpressing NKU-∆minCD. In this work, improved production of mcl-PHA in P. mendocina NK-01 has been achieved by morphology engineering. This work provides an alternative strategy to enhance mcl-PHA accumulation in mcl-PHA-producing strains.





Similar content being viewed by others
References
Anderson DE, Gueiros-Filho FJ, Erickson HP (2004) Assembly dynamics of FtsZ rings in Bacillus subtilis and Escherichia coli and effects of FtsZ-regulating proteins. J Bacteriol 186:5775–5781
Bi E, Lutkenhaus J (1993) Cell division inhibitors SulA and MinCD prevent formation of the FtsZ ring. J Bacteriol 175:1118–1125
Borrero-de Acuña JM, Bielecka A, Häussler S, Schobert M, Jahn M, Wittmann C, Jahn D, Poblete-Castro I (2014) Production of medium chain length polyhydroxyalkanoate in metabolic flux optimized Pseudomonas putida. Microb Cell Factories 13:88
Boyle DS, Khattar MM, Addinall SG, Lutkenhaus J, Donachie WD (1997) ftsW is an essential cell-division gene in Escherichia coli. Mol Microbiol 24:1263–1273
Cabeen MT, Jacobs-Wagner C (2010) The bacterial cytoskeleton. Annu Rev Genet 44:365–392
Chen GQ (2009) A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev 38:2434–2446
Chen GQ, Wu Q (2005) The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 26:6565–6578
Chen Y, Milam S, Erickson H (2012) SulA inhibits assembly of FtsZ by a simple sequestration mechanism. Biochemistry 51:3100–3109
Chung AL, Zeng GD, Jin HL, Wu Q, Chen JC, Chen GQ (2013) Production of medium-chain-length 3-hydroxyalkanoic acids by β-oxidation and phaC operon deleted Pseudomonas entomophila harboring thioesterase gene. Metab Eng 17:23–29
Dajkovic A, Mukherjee A, Lutkenhaus J (2008) Investigation of regulation of FtsZ assembly by SulA and development of a model for FtsZ polymerization. J Biotechnol 190:2513–2526
den Blaauwen T, de Pedro MA, Nguyen-Disteche M, Ayala JA (2008) Morphogenesis of rod-shaped sacculi. FEMS Microbiol Rev 32:321–344
Elhadi D, Lv L, Jiang XR, Wu H, Chen GQ (2016) CRISPRi engineering E. coli for morphology diversification. Metab Eng 38:358–369
Feng J, Gao W, Gu Y, Zhang W, Cao M, Song C, Zhang P, Sun M, Yang C, Wang S (2014) Functions of poly-gamma-glutamic acid (γ-PGA) degradation genes in γ-PGA synthesis and cell morphology maintenance. Appl Microbiol Biotechnol 98:6397–6407
Fontaine P, Mosrati R, Corroler D (2017) Medium chain length polyhydroxyalkanoates biosynthesis in Pseudomonas putida mt-2 is enhanced by co-metabolism of glycerol/octanoate or fatty acids mixtures. Int J Biol Macromol 98:430–435
Gao X, Chen JC, Wu Q, Chen GQ (2011) Polyhydroxyalkanoates as a source of chemicals, polymers, and biofuels. Curr Opin Biotechnol 22:768–774
Gao W, Zhang Z, Feng J, Dang Y, Quan Y, Gu Y, Wang S, Song C (2016) Effects of MreB paralogs on poly-γ-glutamic acid synthesis and cell morphology in Bacillus amyloliquefaciens. FEMS Microbiol Lett 363:fnw187
Gitai Z (2005) The new bacterial cell biology: moving parts and subcellular architecture. Cell 120:577–586
Green MR, Sambrook J (2012) Molecular cloning: a laboratory manual, 4th edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Guo W, Song C, Kong M, Geng W, Wang Y, Wang S (2011a) Simultaneous production and characterization of medium-chain-length polyhydroxyalkanoates and alginate oligosaccharides by Pseudomonas mendocina NK-01. Appl Microbiol Biotechnol 92:791–801
Guo W, Wang Y, Song C, Yang C, Li Q, Li B, Su W, Sun X, Song D, Yang X, Wang S (2011b) Complete genome of Pseudomonas mendocina NK-01, which synthesizes medium-chain-length polyhydroxyalkanoates and alginate oligosaccharides. J Bacteriol 193:3413–3414
Higashitani A, Higashitani N, Horiuchi K (1995) A cell division inhibitor SulA of Escherichia coli directly interacts with FtsZ through GTP hydrolysis. Biochem Biophys Res Commun 209:198–204
Howard M (2004) A mechanism for polar protein localization in bacteria. J Mol Biol 335:655–663
Ivanov V, Mizuuchi K (2010) Multiple modes of interconverting dynamic pattern formation by bacterial cell division proteins. Proc Natl Acad Sci U S A 107:8071–8078
Jiang XR, Chen GQ (2016) Morphology engineering of bacteria for bio-production. Biotechnol Adv 34:435–440
Jiang XR, Wang H, Shen R, Chen GQ (2015) Engineering the bacterial shapes for enhanced inclusion bodies accumulation. Metab Eng 29:227–337
Jiang XR, Yao ZH, Chen GQ (2017) Controlling cell volume for efficient PHB production by Halomonas. Metab Eng 44:30–37
Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RMII, Peterson KM (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176
Li R, Zhang H, Qi Q (2007) The production of polyhydroxyalkanoates in recombinant Escherichia coli. Bioresour Technol 98:2313–2320
Liu Q, Luo G, Zhou XR, Chen GQ (2011) Biosynthesis of poly(3-hydroxydecanoate) and 3-hydroxydodecanoate dominating polyhydroxyalkanoates by beta-oxidation pathway inhibited Pseudomonas putida. Metab Eng 13:11–17
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Loose M, Mitchison TJ (2014) The bacterial cell division proteins FtsA and FtsZ self-organize into dynamic cytoskeletal patterns. Nat Cell Biol 16:38–46
Ma L, Zhang H, Liu Q, Chen J, Zhang J, Chen GQ (2009) Production of two monomer structures containing medium-chain-length polyhydroxyalkanoates by beta-oxidation-impaired mutant of Pseudomonas putida KT2442. Bioresour Technol 100:4891–4894
Margolin W (2005) FtsZ and the division of prokaryotic cells and organelles. Nat Rev Mol Cell Biol 6:862–871
Noda I, Satkowski MM, Dowrey AE, Marcott C (2004) Polymer alloys of Nodax copolymers and poly(lactic acid). Macromol Biosci 4:269–275
Noda I, Green PR, Satkowski MM, Schechtman LA (2005) Preparation and properties of a novel class of polyhydroxyalkanoate copolymers. Biomacromolecules 6:580–586
Ouyang SP, Luo RC, Chen SS, Liu Q, Chung A, Wu Q, Chen GQ (2007) Production of polyhydroxyalkanoates with high 3-hydroxydodecanoate monomer content by fadB and fadA knockout mutant of Pseudomonas putida KT2442. Biomacromolecules 8:2504–2511
Pichoff S, Lutkenhaus J (2001) Escherichia coli division inhibitor MinCD blocks septation by preventing Z-ring formation. J Bacteriol 183:6630–6635
Poblete-Castro I, Binger D, Rodrigues A, Becker J, Martins Dos Santos VA, Wittmann C (2013) In-silico-driven metabolic engineering of Pseudomonas putida for enhanced production of poly-hydroxyalkanoates. Metab Eng 15:113–123
Reddy CS, Ghai R, Rashmi, Kalia VC (2003) Polyhydroxyalkanoates: an overview. Bioresour Technol 87:137–146
Rowlett VW, Margolin W (2013) The bacterial Min system. Curr Biol 23:R553–R556
Song Y, Nikoloff JM, Fu G, Chen J, Li Q, Xie N, Zheng P, Sun J, Zhang D (2016) Promoter screening from Bacillus subtilis in various conditions hunting for synthetic biology and industrial applications. PLoS One 11:e0158447
Tan D, Wu Q, Chen JC, Chen GQ (2014) Engineering Halomonas TD01 for low cost production of polyhydroxyalkanoates. Metab Eng 26:34–47
Tao W, Lv L, Chen GQ (2017) Engineering Halomonas species TD01 for enhanced polyhydroxyalkanoates synthesis via CRISPRi. Microb Cell Factories 16:48
Uehara T, Dinh T, Bernhardt TG (2009) LytM-domain factors are required for daughter cell separation and rapid ampicillin-induced lysis in Escherichia coli. J Bacteriol 191:5094–5107
Uehara T, Parzych KR, Dinh T, Bernhardt TG (2010) Daughter cell separation is controlled by cytokinetic ring-activated cell wall hydrolysis. EMBO J 29:1412–1422
Van den Ent F, Amos LA, Löwe J (2001) Prokaryotic origin of the actin cytoskeleton. Nature 413:39–44
Villanelo F, Ordenes A, Brunet J, Lagos R, Monasterio O (2011) A model for the Escherichia coli FtsB/FtsL/FtsQ cell division complex. BMC Struct Biol 11:28
Wang F, Lee SY (1997) Production of poly(3-hydroxybutyrate) by fed-batch culture of filamentation-suppressed recombinant Escherichia coli. Appl Environ Microbiol 63:4765–4769
Wang S, Arellano-Santoyo H, Combs PA, Shaevitz JW (2010) Actin-like cytoskeleton filaments contribute to cell mechanics in bacteria. Proc Natl Acad Sci U S A 107:9182–9185
Wang Y, Wu H, Jiang XR, Chen GQ (2014) Engineering Escherichia coli for enhanced production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) in larger cellular space. Metab Eng 25:183–193
Wang Y, Zhang C, Gong T, Zuo Z, Zhao F, Fan X, Yang C, Song C (2015) An upp-based markerless gene replacement method for genome reduction and metabolic pathway engineering in Pseudomonas mendocina NK-01 and Pseudomonas putida KT2440. J Microbiol Methods 113:27–33
Wang Y, Zhao F, Fan X, Wang S, Song C (2016) Enhancement of medium-chain-length polyhydroxyalkanoates biosynthesis from glucose by metabolic engineering in Pseudomonas mendocina. Biotechnol Lett 38:313–320
Ward JE Jr, Lutkenhaus J (1985) Overproduction of FtsZ induces minicell formation in E. coli. Cell 42:941–999
Wu H, Fan Z, Jiang X, Chen J, Chen GQ (2016a) Enhanced production of polyhydroxybutyrate by multiple dividing E. coli. Microb Cell Factories 27:15
Wu H, Chen JC, Chen GQ (2016b) Engineering the growth pattern and cell morphology for enhanced PHB production by Escherichia coli. Appl Microbiol Biotechnol 100:9907–9916
Zhou H, Lutkenhaus J (2005) MinC mutants deficient in MinD- and DicB-mediated cell division inhibition due to loss of interaction with MinD, DicB, or a septal component. J Bacteriol 187:2846–2857
Funding
This work was financially supported by the National Natural Science Funding of China (Grant Nos. 31470213, 31570035 and 31670093), the Tianjin Natural Science Funding (Grant Nos. 17JCZDJC32100 and 18JCYBJC24500), and the Postdoctoral Science Funding of China (Grant No. 2018M631729).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 871 kb)
Rights and permissions
About this article
Cite this article
Zhao, F., Gong, T., Liu, X. et al. Morphology engineering for enhanced production of medium-chain-length polyhydroxyalkanoates in Pseudomonas mendocina NK-01. Appl Microbiol Biotechnol 103, 1713–1724 (2019). https://doi.org/10.1007/s00253-018-9546-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9546-8