Abstract
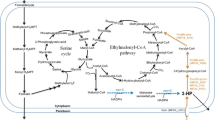
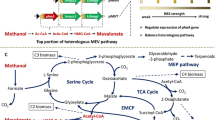
Bio-based production of dicarboxylic acids is an emerging research field with remarkable progress during the last decades. The recently established synthesis of the ethylmalonyl-CoA pathway (EMCP)-derived dicarboxylic acids, mesaconic acid and (2S)-methylsuccinic acid, from the alternative carbon source methanol (Sonntag et al., Appl Microbiol Biotechnol 98:4533–4544, 2014) gave a proof of concept for the sustainable production of hitherto biotechnologically inaccessible monomers. In this study, substantial optimizations of the process by different approaches are presented. Abolishment of mesaconic and (2S)-methylsuccinic acid reuptake from culture supernatant and a productivity increase were achieved by 30-fold decreased sodium ion availability in culture medium. Undesired flux from EMCP into polyhydroxybutyrate (PHB) cycle was hindered by the knockout of polyhydroxyalkanoate synthase phaC which was concomitant with 5-fold increased product concentrations. However, frequently occurring suppressors of strain ΔphaC lost their beneficial properties probably due to redirected channeling of acetyl-CoA. Pool sizes of the product precursors were increased by exploiting the presence of two cobalt-dependent mutases in the EMCP: Fine-tuned growth-limiting cobalt concentrations led to 16-fold accumulation of mesaconyl- and (2S)-methylsuccinyl-CoA which in turn resulted in 6-fold increased concentrations of mesaconic and (2S)-methylsuccinic acids, with a combined titer of 0.65 g/l, representing a yield of 0.17 g/g methanol. This work represents an important step toward an industrially relevant production of ethylmalonyl-CoA pathway-derived dicarboxylic acids and the generation of a stable PHB synthesis negative Methylobacterium extorquens strain.






Similar content being viewed by others
References
Alber B (2011) Biotechnological potential of the ethylmalonyl-CoA pathway. Appl Microbiol Biotechnol 89(1):17–25
Alonso S, Rendueles M, Díaz M (2014) Microbial production of specialty organic acids from renewable and waste materials. Crit Rev Biotechnol :1-17
Anthony C (1982) The biochemistry of methylotrophs. Academic, London
Asadollahi MA, Maury J, Møller K, Nielsen KF, Schalk M, Clark A, Nielsen J (2008) Production of plant sesquiterpenes in Saccharomyces cerevisiae: effect of ERG9 repression on sesquiterpene biosynthesis. Biotechnol Bioeng 99(3):666–677
Bennett BD, Yuan J, Kimball EH, Rabinowitz JD (2008) Absolute quantitation of intracellular metabolite concentrations by an isotope ratio-based approach. Nat Protoc 3(8):1299–311
Bertani G (1951) Studies on lysogenesis I.: the mode of phage liberation by lysogenic Escherichia coli. J Bacteriol 62(3):293–300
Bertau M, Offermanns H, Plass L, Schmidt F, Wernicke H-J (2014) Methanol: the basic chemical and energy feedstock of the future, vol. 1. Springer, Heidelberg
Braunegg G, Sonnleitner B, Lafferty RM (1978) A rapid gas chromatographic method for the determination of poly-β-hydroxybutyric acid in microbial biomass. European J Appl Microbiol Biotechnol 6(1):29–37
Cao Y, Cao Y, Lin X (2011) Metabolically engineered Escherichia coli for biotechnological production of four-carbon 1,4-dicarboxylic acids. J Ind Microbiol Biotechnol 38(6):649–56
Cevallos MA, Encarnación S, Leija A, Mora Y, Mora J (1996) Genetic and physiological characterization of a Rhizobium etli mutant strain unable to synthesize poly-beta-hydroxybutyrate. J Bacteriol 178(6):1646–54
Chistoserdova L, Kalyuzhnaya MG, Lidstrom ME (2009) The expanding world of methylotrophic metabolism. Annu Rev Microbiol 63:477–99
Di Giulio AV, Bauer JN (1982) Fire-retardant anhydride copolymers. US Patent US 4327197
Erb TJ, Berg IA, Brecht V, Müller M, Fuchs G, Alber BE (2007) Synthesis of C5-dicarboxylic acids from C2-units involving crotonyl-CoA carboxylase/reductase: the ethylmalonyl-CoA pathway. Proc Natl Acad Sci USA 104(25):10631–10636
Erb TJ, Rétey J, Fuchs G, Alber BE (2008) Ethylmalonyl-CoA mutase from Rhodobacter sphaeroides defines a new subclade of coenzyme B12-dependent acyl-CoA mutases. J Biol Chem 283(47):32283–32293
Groeneveld M, Detert Oude Weme RGJ, Duurkens RH, Slotboom DJ (2010) Biochemical characterization of the C4-dicarboxylate transporter DctA from Bacillus subtilis. J Bacteriol 192(11):2900–2907
Janausch IG, Zientz E, Tran QH, Kröger A, Unden G (2002) C4-dicarboxylate carriers and sensors in bacteria. BBA - Bioenergetics 1553(1–2):39–56
Jang Y-S, Kim B, Shin JH, Choi YJ, Choi S, Song CW, Lee J, Park HG, Lee SY (2012) Bio-based production of C2–C6 platform chemicals. Biotechnol Bioeng 109(10):2437–2459
Kiefer P, Buchhaupt M, Christen P, Kaup B, Schrader J, Vorholt JA (2009) Metabolite profiling uncovers plasmid-induced cobalt limitation under methylotrophic growth conditions. PLoS ONE 4(11):e7831
Korotkova N, Lidstrom ME (2001) Connection between poly-β-hydroxybutyrate biosynthesis and growth on C1 and C2 compounds in the methylotroph Methylobacterium extorquens AM1. J Bacteriol 183(3):1038–1046
Korotkova N, Chistoserdova L, Lidstrom ME (2002) Poly-β-hydroxybutyrate biosynthesis in the facultative methylotroph Methylobacterium extorquens AM1: identification and mutation of gap11, gap20, and phaR. J Bacteriol 184(22):6174–6181
Kranz RG, Gabbert KK, Locke TA, Madigan MT (1997a) Polyhydroxyalkanoate production in Rhodobacter capsulatus: genes, mutants, expression, and physiology. Appl Environ Microbiol 63(8):3003–9
Kranz RG, Gabbert KK, Madigan MT (1997b) Positive selection systems for discovery of novel polyester biosynthesis genes based on fatty acid detoxification. Appl Environ Microbiol 63(8):3010–3
Lee JW, Kim HU, Choi S, Yi J, Lee SY (2011) Microbial production of building block chemicals and polymers. Curr Opin Biotechnol 22(6):758–767
Litsanov B, Brocker M, Bott M (2012) Toward homosuccinate fermentation: metabolic engineering of Corynebacterium glutamicum for anaerobic production of succinate from glucose and formate. Appl Environ Microbiol 78(9):3325–37
Loos RL, Mijolovic DM, Heimann JW, Szarka ZJL (2012) Polyesters based on 2-methylsuccinic acid. US Patent US 2012/0245256 A1
Marx CJ (2002) Broad-host-range cre-lox system for antibiotic marker recycling in gram-negative bacteria. Biotechniques 33:1062–7
Marx CJ, Lidstrom ME (2001) Development of improved versatile broadhost-range vectors for use in methylotrophs and other Gram-negative bacteria. Microbiology 147(8):2065–2075
Mothes G, Rivera IS, Babel W (1996) Competition between beta-ketothiolase and citrate synthase during poly(beta-hydroxybutyrate) synthesis in Methylobacterium rhodesianum. Arch Microbiol 166(6):405–10
Mothes G, Ackermann JU, Babel W (1998) Regulation of poly(beta-hydroxybutyrate) synthesis in Methylobacterium rhodesianum MB 126 growing on methanol or fructose. Arch Microbiol 169(4):360–3
Muller B, Richard H (2012) Use of a 2-methylsuccinic acid diester derivative as solvent in cosmetic compositions; cosmetic compositions containing the same. World Patent WO2012119861 A2
Ochsner AM, Sonntag F, Buchhaupt M, Schrader J, Vorholt JA (2014) Methylobacterium extorquens: methylotrophy and biotechnological applications. Biotechnol Appl Microbiol 99:517–534
Orita I, Nishikawa K, Nakamura S, Fukui T (2014) Biosynthesis of polyhydroxyalkanoate copolymers from methanol by Methylobacterium extorquens AM1 and the engineered strains under cobalt-deficient conditions. Biotechnol Appl Microbiol 98:3715–3725
Peel D, Quayle JR (1961) Microbial growth on C1 compounds. I. Isolation and characterization of Pseudomonas AM 1. Biochem J 81:465–9
Peters-Wendisch P, Stolz M, Etterich H, Kennerknecht N, Sahm H, Eggeling L (2005) Metabolic engineering of Corynebacterium glutamicum for L-serine production. Appl Environ Microbiol 71(11):7139–7144
Peyraud R, Kiefer P, Christen P, Massou S, Portais J-C, Vorholt JA (2009) Demonstration of the ethylmalonyl-CoA pathway by using 13C metabolomics. Proc Natl Acad Sci USA 106(12):4846–4851
Peyraud R, Schneider K, Kiefer P, Massou S, Vorholt JA, Portais JC (2011) Genome-scale reconstruction and system level investigation of the metabolic network of Methylobacterium extorquens AM1. BMC Syst Biol 5:189
Peyraud R, Kiefer P, Christen P, Portais JC, Vorholt JA (2012) Co-consumption of methanol and succinate by Methylobacterium extorquens AM1. PLoS ONE 7(11):e48271
Povolo S, Tombolini R, Morea A, Anderson AJ, Casella S, Nuti MP (1994) Isolation and characterization of mutants of Rhizobium meliloti unable to synthesize poly-β-hydroxybutyrate. Can J Microbiol 40(10):823–829
Sambrook J (2001) Molecular cloning: a laboratory manual / Joseph Sambrook, David W. Russell. Cold Spring Harbor Laboratory, Cold Spring Harbor
Schlegel HG, Lafferty R, Krauss I (1970) The isolation of mutants not accumulating poly-β-hydroxybutyric acid. Archiv Mikrobiol 71(3):283–294
Schneider K, Peyraud R, Kiefer P, Christen P, Delmotte N, Massou S, Portais JC, Vorholt JA (2012) The ethylmalonyl-CoA pathway is used in place of the glyoxylate cycle by Methylobacterium extorquens AM1 during growth on acetate. J Biol Chem 287(1):757–66
Schrader J, Schilling M, Holtmann D, Sell D, Filho MV, Marx A, Vorholt JA (2009) Methanol-based industrial biotechnology: current status and future perspectives of methylotrophic bacteria. Trends Biotechnol 27(2):107–115
Sonntag F, Buchhaupt M, Schrader J (2014) Thioesterases for ethylmalonyl–CoA pathway derived dicarboxylic acid production in Methylobacterium extorquens AM1. Appl Microbiol Biotechnol 98(10):4533–4544
Teramoto H, Shirai T, Inui M, Yukawa H (2008) Identification of a gene encoding a transporter essential for utilization of C4 dicarboxylates in Corynebacterium glutamicum. Appl Environ Microbiol 74(17):5290–6
Toyama H, Anthony C, Lidstrom ME (1998) Construction of insertion and deletion mxa mutants of Methylobacterium extorquens AM1 by electroporation. FEMS Microbiol Lett 166:1–7
Van Dien SJ, Okubo Y, Hough MT, Korotkova N, Taitano T, Lidstrom ME (2003a) Reconstruction of C(3) and C(4) metabolism in Methylobacterium extorquens AM1 using transposon mutagenesis. Microbiology 149(Pt 3):601–9
Van Dien SJ, Strovas T, Lidstrom ME (2003b) Quantification of central metabolic fluxes in the facultative methylotroph Methylobacterium extorquens AM1 using 13C-label tracing and mass spectrometry. Biotechnol Bioeng 84(1):45–55
Vuilleumier S, Chistoserdova L, Lee M-C, Bringel F, Lajus A, Zhou Y, Gourion B, Barbe V, Chang J, Cruveiller S, Dossat C, Gillett W, Gruffaz C, Haugen E, Hourcade E, Levy R, Mangenot S, Muller E, Nadalig T, Pagni M, Penny C, Peyraud R, Robinson DG, Roche D, Rouy Z, Saenampechek C, Salvignol G, Vallenet D, Wu Z, Marx CJ, Vorholt JA, Olson MV, Kaul R, Weissenbach J, Médigue C, Lidstrom ME (2009) Methylobacterium genome sequences: a reference blueprint to investigate microbial metabolism of C1 compounds from natural and industrial sources. PLoS ONE 4(5):e5584
Witan J, Bauer J, Wittig I, Steinmetz PA, Erker W, Unden G (2012) Interaction of the Escherichia coli transporter DctA with the sensor kinase DcuS: presence of functional DctA/DcuS sensor units. Mol Microbiol 85(5):846–861
Wu L, Mashego MR, van Dam JC, Proell AM, Vinke JL, Ras C, van Winden WA, van Gulik WM, Heijnen JJ (2005) Quantitative analysis of the microbial metabolome by isotope dilution mass spectrometry using uniformly 13C-labeled cell extracts as internal standards. Anal Biochem 336(2):164–71
Yamada M, Wakuda A, Taguchi S (2007a) Morphological change in cellular granule formation of poly[(R)-3-hydroxybutyrate] caused by DNA-binding-related mutations of an autoregulated repressor PhaR. Biosci Biotechnol Biochem 71(6):1572–6
Yamada M, Yamashita K, Wakuda A, Ichimura K, Maehara A, Maeda M, Taguchi S (2007b) Autoregulator protein PhaR for biosynthesis of polyhydroxybutyrate [P(3HB)] possibly has two separate domains that bind to the target DNA and P(3HB): functional mapping of amino acid residues responsible for DNA binding. J Bacteriol 189(3):1118–1127
Yamada M, Takahashi S, Okahata Y, Doi Y, Numata K (2013) Monitoring and kinetic analysis of the molecular interactions by which a repressor protein, PhaR, binds to target DNAs and poly[(R)-3-hydroxybutyrate]. AMB Express 3(1):6
Youn J-W, Jolkver E, Krämer R, Marin K, Wendisch VF (2009) Characterization of the dicarboxylate transporter DctA in Corynebacterium glutamicum. J Bacteriol 191(17):5480–5488
Yu C, Cao Y, Zou H, Xian M (2011) Metabolic engineering of Escherichia coli for biotechnological production of high-value organic acids and alcohols. Appl Microbiol Biotechnol 89(3):573–83
Acknowledgments
This work was funded by the European Union in the context of PROMYSE research project (FP7-KBBE.2011.3.6-04).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sonntag, F., Müller, J.E.N., Kiefer, P. et al. High-level production of ethylmalonyl-CoA pathway-derived dicarboxylic acids by Methylobacterium extorquens under cobalt-deficient conditions and by polyhydroxybutyrate negative strains. Appl Microbiol Biotechnol 99, 3407–3419 (2015). https://doi.org/10.1007/s00253-015-6418-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6418-3