Abstract
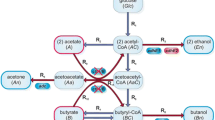
The enzymes β-ketothiolase and citrate synthase from the facultatively methylotrophicMethylobacterium rhodesianum MB 126, which uses the serine pathway, were purified and characterized. The β-ketothiolase had a relatively highK m for acetyl-CoA (0.5 mM) and was strongly inhibited by CoA (K i 0.02 mM). The citrate synthase had a much higher affinity for acetyl-CoA (K m 0.07 mM) and was significantly inhibited by NADH (K i 0.15 mM). The intracellular concentration of CoA metabolites and nucleotides was determined inM. rhodesianum MB 126 during growth on methanol. The level of CoA decreased from about 0.6 nmol (mg dry mass)-1 during growth to the detection limit when poly(β-hydroxybutyrate) (PHB) accumulated. Nearly unchanged intracellular concentrations of NADH, NADPH, and acetyl-CoA of about 0.5, 0.6–0.7, and 1.0 nmol (mg dry mass)-1, respectively, were determined during growth and PHB synthesis. During growth, the β-ketothiolase was almost completely inhibited by CoA, and acetyl-CoA was principally consumed by the citrate synthase. During PHB accumulation, the β-ketothiolase had about 75% of its maximum activity and showed much higher activity than citrate synthase, which at the actual NADH concentration was about 75% inhibited. NADPH concentration was sufficiently high to allow the unlimited activity of acetoacetyl-CoA reductase (K mNADPH 18 μM). PHB synthesis is probably mainly controlled by the CoA concentration inM. rhodesianum MB 126.
Similar content being viewed by others
Abbreviations
- PHB :
-
Poly(β-hydroxybutyrate)
References
Atkinson DE (1968) The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry 7:4030–4034
Babel W, Müller-Kraft G (1979) Regulation der Citrat-Synthase bei methylotropher und chemoorganoheterotropher Ernährung von Bakterien. Z Allg Mikrobiol 19:687–693
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bulthuis BA, Koningstein GM, Stouthamer AH, Van Verseveld HW (1993) The relationship of proton motive force, adenylate energy charge and phosphorylation potential to the specific growth rate and efficiency of energy transduction inBacillus licheniformis under aerobic growth conditions. Antonie Van Leeuwenhoek 63:1–16
Hauche H, Hilger U (1974) Untersuchungen zur Isolierung und Charakterisierung methanassimilierender Bakterien und zum Einfluß einiger Milieufaktoren auf Wachstum und Zellzusammensetzung. PhD Thesis, Akademie der Wissenschaften, Berlin
Haywood GW, Anderson AJ, Chu L, Dawes EA (1988) Characterization of two 3-ketothiolases possessing differing substrate specificities in the polyhydroxyalcanoate organismAlcaligenes eutrophus. FEMS Microbiol Lett 52:91–96
King MT, Reiss PD (1985) Separation and measurement of shortchain Coenzyme A compounds in rat liver by reversed-phase high performance liquid chromatography. Anal Biochem 146:173–179
Kiriukhin MY, Detkov SY, Baev MV, Tsygankov YD (1993) Citrate synthase from the obligate methylotrophMethylobacillus flagellatum. FEMS Microbiol Lett 113:101–106
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee Y, Kim MK, Chang HN, Park YH (1995) Regulation of poly-β-hydroxybutyrate biosynthesis by nicotinamide nucleotide inAlcaligenes eutrophus. FEMS Microbiol Lett 131:35–39
Mansfield DA, Anderson AJ, Naylor LA (1995) Regulation of PHB metabolism inAlcaligenes eutrophus. Can J Microbiol 41:44–49
Mothes G, Babel W (1994)Methylobacterium rhodesianum MB 126 possesses two acetoacetyl-CoA reductases. Arch Microbiol 161:277–280
Mothes G, Babel W (1995)Methylobacterium rhodesianum MB 126 possesses two stereospecific crotonyl-CoA hydratases. Can J Microbiol 41:68–72
Müller RH, Loffhagen N, Babel W (1996) Rapid extraction of (di)nucleotides from bacterial cells and determination by ionpair reversed-phase HPLC. J Microbiol Methods 25:29–35
Nishimura T, Saito T, Tomita K (1978) Purification and properties of β-ketothiolase fromZoogloea ramigera. Arch Microbiol 116:21–27
Otto R (1986) Regulatory and molecular properties of citrate synthases from methylotrophs. FEMS Microbiol Lett 34:191–194
Riis V, Mai W (1988) Gas chromatographic determination of poly-β-hydroxybutyric acid in microbial biomass after hydrochloric acid propanolysis. J Chromatogr 445:285–289
Schulz H (1974) Long chain enoyl coenzyme A hydratase from pig heart. J Biol Chem 249:2704–2709
Senior PJ, Dawes EA (1973) The regulation of poly-β-hydroxybutyrate metabolism inAzoto-bacter beijerinckii. Biochem J 134:225–238
Srere PA (1969) Citrate synthase. In: Lowenstein JM (ed) Methods in enzymology, vol 13. Academic Press, London, pp 3–11
Williams DR, Anderson AJ, Dawes EA (1993) Biosynthesis of polyhydroxyalcanoates inRhodococcus ruber. In: Schlegel HG, Steinbüchel A (eds) Proceedings of the international symposium on bacterial PHA. Goltze-Druck, Gottingen, pp 387–388
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mothes, G., Rivera, I.S. & Babel, W. Competition between β-ketothiolase and citrate synthase during poly(β-hydroxybutyrate) synthesis inMethylobacterium rhodesianum . Arch. Microbiol. 166, 405–410 (1996). https://doi.org/10.1007/BF01682987
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01682987