Abstract
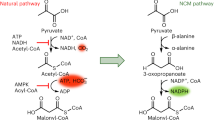
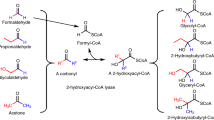
The ethylmalonyl-CoA pathway is central to the carbon metabolism of many α-proteobacteria, like Rhodobacter sphaeroides and Methylobacterium extorquens as well as actinomycetes, like Streptomyces spp. Its function is to convert acetyl-CoA, a central carbon intermediate, to other precursor metabolites for cell carbon biosynthesis. In contrast to the glyoxylate cycle—another widely distributed acetyl-CoA assimilation strategy—the ethylmalonyl-CoA pathway contains many unique CoA-ester intermediates, such as (2R)- and (2S)-ethylmalonyl-CoA, (2S)-methylsuccinyl-CoA, mesaconyl-(C1)-CoA, and (2R, 3S)-methylmalyl-CoA. With this come novel catalysts that interconvert these compounds. Among these unique enzymes is a novel carboxylase that reductively carboxylates crotonyl-CoA, crotonyl-CoA carboxylase/reductase, and (3S)-malyl-CoA thioesterase. The latter represents the first example of a non-Claisen condensation enzyme of the malate synthase superfamily and defines a new class of thioesterases apart from the hotdog-fold and α/β-fold thioesterases. The biotechnological implications of the ethylmalonyl-CoA pathway are tremendous as one looks to tap into the potential of using these new intermediates and catalysts to produce value-added products.




Similar content being viewed by others
References
Alber BE, Spanheimer R, Ebenau-Jehle C, Fuchs G (2006) Study of an alternate glyoxylate cycle for acetate assimilation by Rhodobacter sphaeroides. Mol Microbiol 61:297–309
Anthony C (1982) The biochemistry of methylotrophs. Academic, London
Arai H, Roh JH, Kaplan S (2008) Transriptome dynamics during the transition from anaerobic photosynthesis to aerobic respiration in Rhodobacter sphaeroides 2.4.1. J Bacteriol 190:286–299
Barnes EM, Wakil SJ (1968) Studies of the mechanism of fatty acid synthesis. XIX. Preparation and general properties of palmityl thioesterase. J Biol Chem 243:2955–2962
Bauer CE, Bird TH (1996) Regulatory circuits controlling photosynthesis gene expression. Cell 85:5–8
Benning MM, Wesenberg G, Liu R, Taylor KL, Dunaway-Mariano D, Holden HM (1998) The three-dimensional structure of 4-hydroxybenzoyl-CoA thioesterase from Pseudomonas sp. strain CBS-3. J Biol Chem 273:33572–33579
Berthold CL, Toyota CG, Richards NGJ, Lindqvist Y (2008) Reinvestigation of the catalytic mechanism of formyl-CoA transferase, a class III CoA-transferase. J Biol Chem 283:6519–6529
Buckel W, Dorn U, Semmler R (1981) Glutaconate CoA-transferase from Acidaminococcus fermentas. Eur J Biochem 118:315–321
Buckel W, Kratky C, Golding BT (2006) Stabilization of methylene radicals by cobalamin in coenzyme B12 dependent mutases. Chem Eur J 12:352–362
Cao J, Hang X, Zhao H, Gong W, Dunaway-Mariano D (2009) The mechanism of human hotdog-fold thioesterase 2 (hTHEM2) substarte recognition and catalysis illuminated by a structure and function based analysis. Biochemistry 48:1293–1304
Chan YA, Podevels AM, Kevany BM, Thomas MG (2009) Biosynthesis of polyketide synthase extender units. Nat Prod Rep 26:10–114
Chisterodova L, Kalyuzhnaya MG, Lidstrom ME (2009) The expanding world of methylotrophic metabolism. Annu Rev Microbiol 63:477–499
Chistoserdova L, Lidstrom ME (1996) Molecular characterization of a chromosomal region involved in the oxidation of acetyl-CoA to glyoxylate in the isocitrate-lyase-negative methylotroph Methylobacterium extorquens AM1. Microbiology 142:1459–1468
Chistoserdova L, Chen S-W, Lapidus A, Lidstrom ME (2003) Methylotrophy in Methylobacterium extorquens AM1 from a genomic point of view. J Bacteriol 185:2980–2987
Cho H, Cronan JE (1993) Escherichia coli thioesterase I, molecular cloning and sequencing of the structural gene and identification as a periplasmic enzyme. J Biol Chem 268:9238–9245
Choudhary M, Zabhua X, Fu YX, Kaplan S (2007) Genome analyses of three strains of Rhodobacter sphaeroides: evidence of rapid evolution of chromosome II. J Bacteriol 189:1914–1921
de Koning GJM, Kellerhals M, van Meurs C, Witholt B (1997) A process for the recovery of poly(hydroxyalkanoates) from Pseudomnas. Part 2: process, development and economic evaluation. Bioprocess Eng 17:15–21
Eraso JM, Kaplan S (1994) prrA, a putative response regulator involved in oxygen regulation of photosynthesis gene expression in Rhodobacetr sphaeroides. J Bacteriol 176:32–43
Erb TJ (2009) The ethylmalonyl-CoA pathway: a novel acetyl-CoA assimilation strategy. Doctoral thesis, Albert-Ludwigs-Universität Freiburg
Erb TJ, Berg IA, Brecht V, Müller M, Fuchs G, Alber BE (2007) Synthesis of C5-dicarboxylic acids from C2-units involving crotonyl-CoA carboxylase/reductase: the ethylmalonyl-CoA pathway. Proc Natl Acad Sci USA 104:10631–10636
Erb TJ, Rétey J, Fuchs G, Alber BE (2008) Ethylmalonyl-CoA mutase from Rhodobacter sphaeroides defines a new subclass of coenzyme B12-dependent acyl-CoA mutases. J Biol Chem 283:32283–32293
Erb TJ, Brecht V, Fuchs G, Müller M, Alber BE (2009a) Carboxylation mechanism and stereochemistry of crotonyl-CoA carboxylase/reductase, a carboxylating enoyl-thioester reductase. Proc Natl Acad Sci USA 106:8871–8876
Erb TJ, Fuchs G, Alber BE (2009b) (2S)-Methylsuccinyl-CoA dehydrogenase closes the ethylmalonyl-CoA pathway for acetyl-CoA assimilation. Mol Microbiol 73:992–1008
Erb TJ, Frerichs-Revermann L, Fuchs G, Alber BE (2010) The apparent malate synthase activity of Rhodobacter sphaeroides is due to two paralogous enzymes, (3S)-malyl-coenzyme A (CoA)/β-methylmalyl-CoA lyase and (3S)-malyl-CoA thioesterase. J Bacteriol 192:1249–1258
Eustáquio AS, McGlinchey RP, Liu Y, Hazzard C, Beer LL, Florova G, Alhamadsheh MM, Lechner A, Kale AJ, Kobayashi Y, Reynolds KA, Moore BS (2009) Biosynthesis of the salinosporamide A polyketide synthase substrate chloroethylmalonyl-coenzyme A from S-adenosyl-l-methionine. Proc Natl Acad Sci USA 106:12295–12300
Fleck CB, Brock M (2008) Characterization of an acyl-CoA:carboxylate CoA-transferase from Aspergillus nidulans involved in propionyl-CoA detoxification. Mol Microbiol 68:642–656
Fuchs G (1999) Biosynthesis of building blocks, chapter 7. In: Lengeler JW, Drews G, Schlegel HG (eds) Biology of the prokaryotes. Thieme, Stuttgart, pp 114–116
Gibson JL, Tabita FR (1993) Nucleotide sequence and functional analysis of CbbR, a positive regulator of the Calvin cycle operons of Rhodobacter sphaeroides. J Bacteriol 175:5778–5784
Goulding CW, Bowers PM, Segelke B, Lekin T, Kim C-Y, Terwilliger TC, Eisenberg D (2007) The structure and computational analysis of Mycobacterium tuberculosis protein CitE suggest a novel enzymatic function. J Mol Biol 365:275–283
Gu L, Wang B, Kulkarni A, Geders TW, Gri RV, Gerwick L, Håkansson K, Wipf P, Smith JL, Gerwick WH, Sherman DH (2009) Metamorphic enzyme assembly in polyketide diversification. Nature 459:731–735
Heider J (2001) A new family of CoA-transferases. FEBS Lett 509:345–349
Howard BR, Endrizzi JA, Remington SJ (2000) Crystal structure of Escherichia coli malate synthase G complexed with magnesium and glyoxylate at 2.0 Å resolution: mechanistic implications. Biochemistry 39:3156–3168
Ind AC, Porter SL, Brown MT, Byles ED, de Beyer JA, Godfrey SA, Armitage JP (2009) Inducible-expression plasmid for Rhodobacter sphaeroides and Paracoccus denitrificans. Appl Environ Microbiol 75:6613–6615
Joshi HM, Tabita FR (1996) A global two component signal transduction system that integrates the control of photosynthesis, carbon dioxide assimilation, and nitrogen fixation. Proc Natl Acad Sci USA 93:14515–14520
Kapritchkoff FM, Viotti AP, Alli RCP, Zuccolo M, Pradella JGC, Maiorano AE, Miranda EA, Bonomi A (2006) Enzymatic recovery and purification of ployhydroxybutyrate produced by Ralstonia eutropha. J Biotechnol 122:453–462
Kim BS, Cropp TA, Beck BJ, Sherman DH, Reynolds KA (2002) Biochemical evidence for an editing role of thioesterase II in the biosynthesis of the polyketide pikromycin. J Biol Chem 277:48028–48034
Koglin A, Löhr F, Bernhard F, Rogov VV, Frueh DP, Strieter ER, Mofid MR, Güntert P, Wagnaer G, Walsh CT, Marahiel MA, Dötsch V (2008) Structural basis for the selectivity of the external thioesterase of the surfactin synthetase. Nature 454:907–911
Kornberg HL, Krebs HA (1957) Synthesis of cell constituents from C2-units by a modified tricarboxylic acid cycle. Nature 179:988–991
Kornberg HL, Phizackerley PJR, Sadler JR (1958) Synthesis of cell constituents from acetate by Escherichia coli. Biochem J 72:32P–33P
Korotkova N, Lidstrom ME (2001) Connection between ploy-β-hydroxybutyrate biosynthesis and growth on C1 and C2 compounds in the methylotroph Methylobacterium extorquens AM1. J Bacteriol 183:1038–1046
Korotkova N, Lidstrom ME (2004) MeaB is a component of the methylmalonyl-CoA mutase complex required for protection of the enzyme from inactivation. J Biol Chem 279:13652–13658
Korotkova N, Chistoserdova L, Kuska V, Lidstrom ME (2002) Glyoxylate regeneration pathway in the methylotroph Methylobacterium extorquens AM1. J Bacteriol 184:1750–1758
Korotkova N, Lidstrom ME, Chistoserdova L (2005) Identification of genes involved in the glyoxylate regeneration cycle in Methylobacterium extorquens AM1, including two genes, meaC and meaD. J Bacteriol 187:1523–1526
Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM, Peterson KM (1995) Four derivatives of the braod-host range cloning vector pBBR1MCS carrying different antibiotic-resistance cassettes. Gene 166:175–176
Large PJ, Peel D, Quayle JR (1961) Microbial growth on C1 compounds. Biochem J 81:470–480
Larsen RA, Wilson MM, Guss AM, Metcalf WW (2002) Genetic analysis of pigmant biosynthesis in Xanthobacter autrophicus Py2 using a new, highly efficient transposon mutagenesis system that is functional in a wide variety of bacteria. Arch Microbiol 178:193–201
Li J, Derewenda U, Dauetr Z, Smith S, Derewenda ZS (2000) Crystal structure of the Escherichia coli thioesterase II, a homolog of the human Nef binding enzyme. Nat Struct Biol 7:555–559
Lim S-K, Kim SJ, Cha SH, Oh Y-K, Rhee H-J, Kim M-S, Lee JK (2009) Complete genome sequence of Rhodobacter sphaeroides KD131. J Bacteriol 191:1118–1119
Lin CY, Smith S (1978) Properties of the thioesterase component obtained by limited trypsinization of the fatty acid synthetase multienzyme complex. J Biol Chem 253:1954–1962
Liu Y, Hazzard C, Eustáquio AS, Reynolds KA, Moore BS (2009) Biosynthesis of salinosporamides from α, β-unsaturated fatty acids: implications for extending polyketide synthase diversity. J Am Chem Soc 131:10376–10377
Lo YC, Lin SC, Shaw JF, Liaw YC (2003) Crystal structure of Escherichia coli thioesterase I/protease I/lysophospholipase L1: consensus sequence blocks constitute the catalytic center of SGNH-hydrolases through a conserved hydrogen bond network. J Mol Biol 330:539–551
Marx A, Poetter M, Buchholz S, May A, Siegert H, Alber B, Fuchs G, Eggerling L (2007) Microbiological production of 3-hydroxybutyric acid. WO/2007/141208
Meister M, Saum S, Alber BE, Fuchs G (2005) L-Malyl-coenzyme A/β-methylmalyl-coenzyme A lyase is involved in acetate assimilation of the isocitrate lyase-negative bacterium Rhodobacter capsulatus. J Bacteriol 187:1415–1425
Neidhardt FC, Ingraham JL, Schaechter M (1990) In: Physiology of the bacterial cell. Chapter 5: biosynthesis and fueling. Sinauer, Sunderland, pp 133–172
Peyraud R, Kiefer P, Christen P, Massou S, Portais JC, Vorholt JA (2009) Demonstration of the ethylmalonyl-CoA pathway by using 13C metabolomics. Proc Nat Acad Sci USA 106:4846–4851
Pickart CM, Jencks WP (1979) Formation of stable anhydrides from CoA transferase and hydroxamic acids. J Biol Chem 254:9120–9129
Quandt J, Hynes MF (1993) Versatile suicide vectors which allow direct selection for gene replacement in Gram-negative bacteria. Gene 127:15–21
Rétey J (1990) Enzymatic reaction selectively by negative catalysis or how do enzymes deal with highly reactive intermediates. Angew Chem Int Ed 29:355–361
Roh JH, Smith WE, Kaplan S (2004) Effects of oxygen and light intensity on transcriptome expression in Rhodobacter sphaeroides 2.4.1. Redox active gene epression profile. J Biol Chem 279:9146–9155
Rohwerder T, Müller RH (2010) Biosynthesis of 2-hydroxyisobutyric acid (2-HIBA) from renewable carbon. Microb Cell Fact 25:13
Schäfer A, Tauch A, Jager W, Kalinowski J, Thierbach G, Puhler A (1994) Small mobilizable multipurpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73
Schrader J, Schilling M, Holtmann D, Sell D, Filho MV, Marx A, Vorholt JA (2008) Methanol-based industrial biotechnology: current status and future perspectives of methylotrophic bacteria. Trends Biotechnol 27:107–115
Schwarzer D, Mootz HD, Linne U, Marahiel MA (2002) Regeneration of misprimed nonribosomal peptide synthases by type II thioesterases. Proc Natl Acad Sci USA 99:14083–14088
Sganga MW, Bauer CE (1992) Regulatory factors controlling photosynthetic reaction center and light-harvesting gene expression in Rhodobacter capsulatus. Cell 68:945–954
Smith LM, Meijer WG, Dijkuizen L, Goodwin PM (1996) A protein having similarity with methylmalonyl-CoA mutase is required for the assimilation of methanol and ethanol by Methylobacterium extorquens AM1. Microbiology 142:675–684
Thoden JB, Holden HM, Zhuang Z, Dunaway-Mariano D (2002) X-ray crystallographic analyses of inhibitor and substrate complexes of wild-type and mutant 4-hydroxybenzoyl-CoA thioesterase. J Biol Chem 277:27468–27476
Thoden JB, Zhuang Z, Dunaway-Mariano D, Holden HM (2003) The structure of 4-hydroxybenzoyl-CoA thioesterase from Arthrobacter sp. strain SU. J Biol Chem 278:43709–43716
Vlasie MD, Banerjee R (2004) When a spectator turns killer: suicidal electron transfer from cobalamin in methylmalonyl-CoA mutase. Biochemistry 43:8410–8417
Wang XD, Falcone L, Tabita FR (1993) Reductive pentose phosphate-independent CO2 fixation in Rhodobacter sphaeroides and evidence that ribulose bisphosphate carboxylase/oxygenase activity serves to maintain the redox balance of the cell. J Bacteriol 175:3372–3379
Zarzycki J, Schlichting A, Strychalsky N, Müller M, Alber BE, Fuchs G (2008) Mesaconyl-coenzyme A hydratase, a new enzyme of two central carbon metabolic pathways in bacteria. J Bacteriol 190:1366–1374
Zhuang Z, Gartemann K-H, Eichnelaub R, Dunaway-Mariano (2003) Characterization of the 4-hydroxybenzoyl-coenzyme A tioesterase from Arthrobacter sp. strain SU. Appl Environ Microbiol 69:2707–2711
Acknowledgments
This research has been supported by the Deutsche Forschungsgemeinschaft (AL677/1-1) and by Evonik-Degussa GmbH in the past and is currently generously funded by the National Science Foundation (MCB0842892). I would like to thank Chuck Daniels for very helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alber, B.E. Biotechnological potential of the ethylmalonyl-CoA pathway. Appl Microbiol Biotechnol 89, 17–25 (2011). https://doi.org/10.1007/s00253-010-2873-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2873-z