Abstract
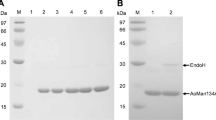
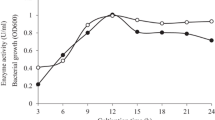
Thermophilic β-mannanases are of increasing importance for wide industrial applications. In the current study, gene cloning, functional expression in Pichia pastoris, and characterization of a thermophilic β-mannanase (Man5A) from thermophilic Talaromyces leycettanus JCM12802 are reported. Deduced Man5A exhibits the highest identity with a putative β-mannanase from Talaromyces stipitatus ATCC10500 (70.3 %) and is composed of an N-terminal signal peptide, a fungal-type carbohydrate-binding module (CBM) of family 1, and a catalytic domain of glycosyl hydrolase (GH) family 5 at the C-terminus. Two recombinant proteins with different glycosylation levels, termed Man5A1 (72 kDa) and Man5A2 (60 kDa), were identified after purification. Both enzymes were thermophilic, exhibiting optimal activity at 85–90 °C, and were highly stable at 70 °C. Man5A1 and Man5A2 had a pH optimum of 4.5 and 4.0, respectively, and were highly stable over the broad pH range of 3.0–10.0. Most metal ions and sodium dodecyl sulfate (SDS) had no effect on the enzymatic activities. Man5A1 and Man5A2 exhibited high specific activity (2,160 and 1,800 U/mg, respectively) when using locust bean gum as the substrate. The CBM1 and two key residues D191 and R286 were found to affect Man5A thermostability. Man5A displays a classical four-site-binding mode, hydrolyzing mannooligosaccharides into smaller units, galactomannan into mannose and mannobiose, and glucomanman into mannose, mannobiose, and mannopentaose, respectively. All these properties make Man5A a good candidate for extensive applications in the bioconversion, pulp bleaching, textile, food, and feed industries.





Similar content being viewed by others
References
Anderson L, Hägglund P, Stoll D, Lo Leggio L, Drakenberg T, Stålbrand H (2008) Kinetics and stereochemistry of the Cellulomonas fimi β-mannanase studied using 1H-NMR. Biocatal Biotransform 26:86–95
Aspeborg H, Coutinho PM, Wang Y, Brumer H, Henrissat B (2012) Evolution, substrate specificity and subfamily classification of glycoside hydrolase family 5 (GH5). BMC Evol Biol 12:186
Benech R-O, Li X, Patton D, Powlowski J, Storms R, Bourbonnais R, Paice M, Tsang A (2007) Recombinant expression, characterization, and pulp prebleaching property of a Phanerochaete chrysosporium endo-β-1,4-mannanase. Enzym Microb Technol 41:740–747
Bien-Cuong D, Thi-Thu D, Berrin JG, Haltrich D, Kim-Anh T, Sigoillot JC, Yamabhai M (2009) Cloning, expression in Pichia pastoris, and characterization of a thermostable GH5 mannan endo-1,4-β-mannosidase from Aspergillus niger BK01. Microb Cell Factories 8:59
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cai H, Shi P, Luo H, Bai Y, Huang H, Yang P, Yao B (2011) Acidic β-mannanase from Penicillium pinophilum C1: cloning, characterization and assessment of its potential for animal feed application. J Biosci Bioeng 112:551–557
Chauhan PS, Puri N, Sharma P, Gupta N (2012) Mannanases: microbial sources, production, properties and potential biotechnological applications. Appl Microbiol Biotechnol 93:1817–1830
Chen X, Cao Y, Ding Y, Lu W, Li D (2007) Cloning, functional expression and characterization of Aspergillus sulphureus β-mannanase in Pichia pastoris. J Biotechnol 128:452–461
Comfort DA, Chhabra SR, Conners SB, Chou CJ, Epting KL, Johnson MR, Jones KL, Sehgal AC, Kelly RM (2004) Strategic biocatalysis with hyperthermophilic enzymes. Green Chem 6:459–465
Couturier M, Roussel A, Rosengren A, Leone P, Stålbrand H, Berrin JG (2013) Structural and biochemical analyses of glycoside hydrolase families 5 and 26 β-(1,4)-mannanases from Podospora anserina reveal differences upon manno-oligosaccharide catalysis. J Biol Chem 288:14624–14635
Davé V, McCarthy SP (1997) Review of konjac glucomannan. J Environ Polym Degr 5:237–241
Fonseca-Maldonado R, Vieira DS, Alponti JS, Bonneil E, Thibault P, Ward RJ (2013) Engineering the pattern of protein glycosylation modulates the thermostability of a GH11 xylanase. J Biol Chem 288:25522–25534
Gilbert HJ, Stålbrand H, Brumer H (2008) How the walls come crumbling down: recent structural biochemistry of plant polysaccharide degradation. Curr Opin Plant Biol 11:338–348
Hall M, Rubin J, Behrens SH, Bommarius AS (2011) The cellulose-binding domain of cellobiohydrolase Cel7A from Trichoderma reesei is also a thermostabilizing domain. J Biotechnol 155:370–376
Han Y, Dodd D, Hespen CW, Ohene-Adjei S, Schroeder CM, Mackie RI, Cann IK (2010) Comparative analyses of two thermophilic enzymes exhibiting both β-1,4 mannosidic and β-1,4 glucosidic cleavage activities from Caldanaerobius polysaccharolyticus. J Bacteriol 192:4111–4121
Helenius A, Aebi M (2004) Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem 73:1019–1049
Hogg D, Woo EJ, Bolam DN, McKie VA, Gilbert HJ, Pickersgill RW (2001) Crystal structure of mannanase 26A from Pseudomonas cellulosa and analysis of residues involved in substrate binding. J Biol Chem 276:31186–31192
Hogg D, Pell G, Dupree P, Goubet F, Martin-Orue S, Armand S, Gilbert H (2003) The modular architecture of Cellvibrio japonicus mannanases in glycoside hydrolase families 5 and 26 points to differences in their role in mannan degradation. Biochem J 371:1027–1043
Hsiao YM, Liu YF, Fang MC, Tseng YH (2010) Transcriptional regulation and molecular characterization of the manA gene encoding the biofilm dispersing enzyme mannan endo-1,4-β-mannosidase in Xanthomonas campestris. J Agr Food Chem 58:1653–1663
Jiang Z, Wei Y, Li D, Li L, Chai P, Kusakabe I (2006) High-level production, purification and characterization of a thermostable β-mannanase from the newly isolated Bacillus subtilis WY34. Carbohydr Polym 66:88–96
Kim DY, Ham SJ, Lee HJ, Cho HY, Kim JH, Kim YJ, Shin DH, Rhee YH, Son KH, Park HY (2011) Cloning and characterization of a modular GH5 β-1, 4-mannanase with high specific activity from the fibrolytic bacterium Cellulosimicrobium sp. strain HY-13. Bioresour Technol 102:9185–9192
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Le Nours J, Anderson L, Stoll D, Stålbrand H, Lo Leggio L (2005) The structure and characterization of a modular endo-β-1,4-mannanase from Cellulomonas fimi. Biochemistry 44:12700–12708
Liu YG, Whittier RF (1995) Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics 25:674–681
Lu H, Zhang H, Shi P, Luo H, Wang Y, Yang P, Yao B (2013) A family 5 β-mannanase from the thermophilic fungus Thielavia arenaria XZ7 with typical thermophilic enzyme features. Appl Microbiol Biotechnol 97:8121–8128
Lu H, Luo H, Shi P, Huang H, Meng K, Yang P, Yao B (2014) A novel thermophilic endo-β-1, 4-mannanase from Aspergillus nidulans XZ3: functional roles of carbohydrate-binding module and Thr/Ser-rich linker region. Appl Microbiol Biotechnol 98:2155–2163
Luo H, Wang Y, Wang H, Yang J, Yang Y, Huang H, Yang P, Bai Y, Shi P, Fan Y (2009) A novel highly acidic β-mannanase from the acidophilic fungus Bispora sp. MEY-1: gene cloning and overexpression in Pichia pastoris. Appl Microbiol Biotechnol 82:453–461
Luo H, Wang K, Huang H, Shi P, Yang P, Yao B (2012) Gene cloning, expression, and biochemical characterization of an alkali-tolerant β-mannanase from Humicola insolens Y1. J Ind Microbiol Biotechnol 39:547–555
Ma Y, Xue Y, Dou Y, Xu Z, Tao W, Zhou P (2004) Characterization and gene cloning of a novel β-mannanase from alkaliphilic Bacillus sp. N16–5. Extremophiles 8:447–454
Mikkonen KS, Yadav MP, Cooke P, Willför S, Hicks KB, Tenkanen M (2008) Films from spruce galactoglucomannan blended with poly (vinyl alcohol), corn arabinoxylan, and konjac glucomannan. BioResources 3:178–191
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Mizutani K, Fernandes VO, Karita S, Luís AS, Sakka M, Kimura T, Jackson A, Zhang X, Fontes CM, Gilbert HJ (2012) Influence of a mannan binding family 32 carbohydrate binding module on the activity of the appended mannanase. Appl Environ Microbiol 78:4781–4787
Moreira L (2008) An overview of mannan structure and mannan-degrading enzyme systems. Appl Microbiol Biotechnol 79:165–178
Naganagouda K, Salimath P, Mulimani V (2009) Purification and characterization of endo-β-1,4 mannanase from Aspergillus niger for application in food processing industry. J Microbiol Biotechnol 19:1184–1190
Oda Y, Komaki T, Tonomura K (1993) Purification and properties of extracellular β-mannanases produced by Enterococcus casseliflavus FL2121 isolated from decayed Konjac. J Ferment Bioeng 76:14–18
Qi F, Zhang W, Zhang F, Chen G, Liu W (2014) Deciphering the effect of the different N-glycosylation sites on the secretion, activity, and stability of cellobiohydrolase I from Trichoderma reesei. Appl Environ Microbiol 80:3962–3971
Sabini E, Schubert H, Murshudov G, Wilson KS, Siika-Aho M, Pentillä M (2000) The three-dimensional structure of a Trichoderma reesei mannanase from glycoside hydrolase family 5. Acta Crystallogr D Biol Crystallogr 56:3–13
Scheller HV, Ulvskov P (2010) Hemicelluloses. Annu Rev Plant Biol 61:263–289
Shallom D, Shoham Y (2003) Microbial hemicellulases. Curr Opin Microbiol 6:219–228
Stålbrand H, Siika-aho M, Tenkanen M, Viikari L (1993) Purification and characterization of two β-mannanases from Trichoderma reesei. J Biotechnol 29:229–242
Tenkanen M, Makkonen M, Perttula M, Viikari L, Teleman A (1997) Action of Trichoderma reesei mannanase on galactoglucomannan in pine kraft pulp. J Biotechnol 57:191–204
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Thongekkaew J, Ikeda H, Iefuji H (2012) Increases thermal stability and cellulose-binding capacity of Cryptococcus sp. S-2 lipase by fusion of cellulose binding domain derived from Trichoderma reesei. Biochem Biophys 420:183–187
Wei W, Chen L, Zou G, Wang Q, Yan X, Zhang J, Wang C, Zhou Z (2013) N-glycosylation affects the proper folding, enzymatic characteristics and production of a fungal β-glucosidase. Biotechnol Bioproc Eng 110:3075–3084
Zhang Y, Ju J, Peng H, Gao F, Zhou C, Zeng Y, Xue Y, Li Y, Henrissat B, Gao G (2008) Biochemical and structural characterization of the intracellular mannanase AaManA of Alicyclobacillus acidocaldarius reveals a novel glycoside hydrolase family belonging to clan GH-A. J Biol Chem 283:31551–31558
Zhang M, Chen X, Zhang Z, Sun C, Chen L, He H, Zhou B, Zhang Y (2009) Purification and functional characterization of endo-β-mannanase MAN5 and its application in oligosaccharide production from konjac flour. Appl Microbiol Biotechnol 83:865–873
Zhao W, Zheng J, Zhou H (2011) A thermotolerant and cold-active mannan endo-1,4-β-mannosidase from Aspergillus niger CBS513.88: constitutive overexpression and high-density fermentation in Pichia pastoris. Bioresour Technol 102:7538–7547
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31172235) and the National High-Tech Research and Development Program of China (863 Program, 2012AA022208) and the National Science and Technology Support Program of China (2011BADB02) and the China Modern Agriculture Research System (CARS-42).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 550 kb)
Rights and permissions
About this article
Cite this article
Wang, C., Luo, H., Niu, C. et al. Biochemical characterization of a thermophilic β-mannanase from Talaromyces leycettanus JCM12802 with high specific activity. Appl Microbiol Biotechnol 99, 1217–1228 (2015). https://doi.org/10.1007/s00253-014-5979-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5979-x