Abstract
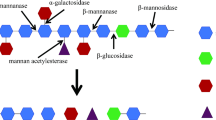
Mannans are the major constituents of the hemicellulose fraction in softwoods and show widespread distribution in plant tissues. The major mannan-degrading enzymes are β-mannanases, β-mannosidases and β-glucosidases. In addition to these, other enzymes such as α-galactosidases and acetyl mannan esterases, are required to remove the side chain substituents. The mannanases are known to be produced by a variety of bacteria, fungi, actinomycetes, plants and animals. Microbial mannanases are mainly extracellular and can act in wide range of pH and temperature because of which they have found applications in pulp and paper, pharmaceutical, food, feed, oil and textile industries. This review summarizes the studies on mannanases reported in recent years in terms of important microbial sources, production conditions, enzyme properties, heterologous expression and potential industrial applications.


Similar content being viewed by others
References
Abdeshanian P, Samat N, Hamid AA, Yusoff WMW (2009) Utilization of palm kernel cake for production of β-mannanase by Aspergillus niger FTCC 5003 in solid state fermentation using an aereated column bioreactor. J Microbiol Biotechnol 37:103–109
Adenmark P, Varga A, Medve J, Harjunpaa V, Drakenberg T, Terneld F, Stalbrand H (1998) Softwood hemicelluloses-degrading enzymes from Aspergillus niger: purification and properties of a β-mannanase. J Biotechnol 63:199–210
Adibmoradi M, Mehri M (2007) Effects of β-mannanase on broiler performance and gut morphology. 16th European Symposium on Poultry Nutrition, Stasburg, France, pp 471–47
Agrawal P, Verma D, Daniell H (2011) Expression of Trichoderma reesei β-mannanase in tobacco chloroplasts and its utilization in lignocellulosic woody biomass hydrolysis. PLoS One 6(12):e29302. doi:10.1371/journal.pone.0029302
Ahmed S, Riaz S, Jamil A (2009) Molecular cloning of fungal xylanase: an overview. Appl Microbiol Biotechnol 84:19–35
Alam NH, Meier R, Schneider H, Sarker SA, Bardhan PK, Mahalanabis D, Fuchs GJ, Gyr N (2000) Partially hydrolyzed guar gum-supplemented oral rehydration solution in the treatment of acute diarrhea in children. J Ped Gas Nut 31:503–507
Aziz SA, Ong LGA, Hassan MA, Karim MIA (2008) Production parameters optimization of mannanse production from Aspergillus niger FTCC 5003 using palm kernel cake as carbon source. Asi J Biochem 3(5):297–307
Benech RO, Li X, Patton D, Powlowski J, Storms R, Bourbonnais R, Paice M, Tsang A (2007) Recombinant expression, characterization, and pulp prebleaching property of a Phanerochaete chrysosporium endo-β-1,4-mannanase. Enzyme Microb Technol 41:740–747
Bettiol JLP, Boutique JP, Gualco LMP, Johnston JP (2000) Nonaqueous liquid detergent compositions comprising a borate releasing compound and a mannanase. Patent EP1059351
Bhoria P, Singh G, Hoondal GS (2009) Optimization of mannanase production from Streptomyces sp. PG-08-03 in submerged fermentation. Bioresources 4(3):1130–1138
Blibech M, Ghorbel RE, Fakhfakh I, Ntarima P, Piens K, Bacha AB, Chaabouni SE (2010) Purification and characterization of a low molecular weight of β-mannanases from Penicillium occitanis Pol6. App Biochem Biotechnol 160:1227–1240
Blibech M, Ghorbel RE, Chaari F, Dammak I, Bhiri F, Neifar M, Chaabouni SE (2011) Improved mannanase production from Penicillium occitanis by fed-batch fermentation using acacia seeds. ISRN Microbiol. doi:10.5402/2011/938347
Bo X, Lei D, Xiang-hua T, Jun-jun L, Yue-lin M, Yun-juan Y (2009) Characterization of 6 Bacillus subtilis β-mannanases and their genes. African J Biotechnol 8(18):4316–4324
Boraston AB, Bolam DN, Gilbert HJ, Davies GJ (2004) Carbohydrate-binding module: fine tuning polysaccharide recognition. J Biochem 382:769–781
Cai H, Shi P, Luo H, Bai Y, Huang H, Yang P, Yao B (2011a) Acidic β-mannanase from Penicillium pinophilum C1: cloning, characterization and assessment of its potential for animal feed application. J Biosci Bioeng 112:551–557. doi:10.1016/j.jbiosc.2011.08.018
Cai H, Shi P, Huang H, Luo H, Bai Y, Yang P, Meng K, Yao B (2011b) An acidic β-mannanase from Penicillium sp. C6: gene cloning and over-expression in Pichia pastoris. World J Microbiol Biotechnol 27:2813–2819
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The Carbohydrate-Active Enzymes database (Cazy): an expert resoureces of glycogenomics. Nucleic Acids Res 37:233–238
Cao S, Hu Z (2011) Characterization the expressed enzyme efficient hydrolysis of mannan and heteromannan in yeast. Micro Biochem Technol 3(1):001–005
Cartmell A, Topakas E, Duros VMA, Suits MDL, Davies GJ, Gilbert HJ (2008) The Cellvibrio japonicus mannanase CjMan26C displays a unique exo-mode of action that is conferred by subtle changes to the distal region of the active sites. J Biochem 283(49):34403–34413
Chandra MRS, Lee YS, Park IH, Zhou Y, Kim KK, Choi YL (2011) Isolation, purification and characterization of a thermostable β-mannanase from Paenibacillus sp. DZ3. J Korean Soc. App. Biol Chem 54(3):325–331
Chen BK, Diosady LL (2003) Enzymatic aqueous processing of coconuts. Int Appl Sci Engg 1:55–61
Chen X, Cao Y, Ding Y, Lu W, Li D (2007) Cloning, functional expression and characterization of Aspergillus sulphureus beta-mannanase in Pichia pastoris. J Biotechnol 128(3):452–461
Chen X, Lu W, Cao Y, Li D (2008) Prokaryotic expression, purification and characterization of Aspergillus sulphureus β-mannanase and site directed mutagenesis of the catalytic residues. Appl Biochem Biotechnol 149:139–144
Cho KM, Math RK, Hong SY, Islam SMA, Kim JO, Hong SJ, Kim H, Yun HD (2008) Changes in the activity of the multifunctional β-glycosyl hydrolase (Cel44C-Man26A) from Paenibacillus polymyxa by removal of the C-terminal region to minimum size. Biotechnol Lett 30:1061–1068
Comfort DA, Swapnil R, Chhabra SR, Conners SB, Chou CJ, Epting KL (2004) Strategic biocatalysis with hyperthermophilic enzymes. Green Chem 6:459–465
Daskiran MRG, Teeter DW, Fodge D, Hsiao HY (2004) An evaluation of endo-ß-D-mannanase (Hemicell) effects on broiler performance and energy use in diets varying in ß-mannan content. Poult Sci 83:662–668
Davies GJ, Wilson KS, Henrissat B (1997) Nomenclature for sugar-binding subsites in glycosyl hydrolases. Biochemistry 321:557–559
Dhawan S, Kaur J (2007) Microbial mannanases: an overview of production and applications. Crit Rev Biotechnol 27(4):197–216
Duruksu G, Ozturk B, Biely P, Ogel ZB (2009) Cloning, expression and characterization of endo-β-1,4-mannanase from Aspergillus fumigates in Aspergillus sojae and Pichia pastoris. Biotechnol Prog 25:271–276
Eneyskaya EV, Sundqvist G, Golubev AM, Ibatullin FM, Ivanen DR, Shabalin KA, Brumer H, Kulminskaya AA (2009) Transglycosylating and hydrolytic activities of the β-mannosidase from Trichoderma reesei. Biochimie 91:632–638
Fattah AAF, Hashem AM, Ismail AMS, Refai EMA (2009) Purification and some properties of β-mannanase from Aspergillus oryzae NRRL 3448. J App Sci Res 5(12):2067–2073
Fu Y, Jeong SH, Kim J, Callihan JA, Park K, Pai CM (2006) Mannose-based fast dissolving tablets. Patent US20060134195A1
Fu X, Huang H, Liu P, Lin L, Wu G, Li C, Feng C, Hong H (2009) Cloning and characterization of a novel mannananses from Paenibacillus sp. BME-14. J Microbiol Biotechnol 20(3):518–524
Gilbert HJ (2010) The biochemistry and structural biology of plant cell wall deconstruction. Plant Physio 153:444–455
Gilbert HJ, Stalbrand H, Brumer H (2008) How the walls come crumbling down: recent structural biochemistry of plant polysaccharide degradation. Curr Opin Plant Biol 11:338–348
Gubitz GM, Lischnig T, Stebbing D, Saddler JN (1997) Enzymatic removal of hemicellulose from dissolving pulps. Biotechnol Lett 19:491–495
Hagglund P, Eriksson T, Collen A, Nerinckx W, Claeyssens M, Stalbrand H (2003) A cellulose-binding module of the Trichoderma reesei β-mannanase Man5A increases the mannan hydrolysis of complex substrates. J Biotechnol 101:37–48
Harris PJ, Stone BA (2008) Chemistry and molecular organization of plant cell walls. In: Himmel ME (ed) Biomass recalcitrance. Blackwell, Oxford, pp 60–93
He X, Liu N, Zhang Z, Zhang B, Ma Y (2008) Inducible and constitutive expression of a novel thermostable alkaline β-mannanase from alkalophilic Bacillus sp. N16-5 in Pichia pastoris and characterization of the recombinant enzyme. Enzyme Microb Technol 43:13–18
Hogg D, Woo EJ, Bolam DN, McKie VA, Gilbert HJ, Pickersgill RW (2001) Crystal structure of mannanase 26 A from Pseudomonas cellulosa and analysis of residues involved in substrate binding. J Bio Chem 276:31186–31192
Hsiao YM, Liu YF, Fang MC, Tseng YH (2010) Transcriptional regulation and molecular characterization of the manA gene encoding the biofilm dispersing enzyme mannan endo-1,4-β-mannosidase in Xanthomonas campestris. J Agric Food Chem 58:1653–1663
Jeon SD, Yu KO, Kim SW, Han SO (2011) A cellulolytic complex from Clostridium cellulovorans consisting of mannanase B and endoglucanase E has synergistic effects on galactomannan degradation. Appl Microbiol Biotechnol 90:565–572
Jørgensen H, Sanadi AR, Felby C, Lange NEK, Fischer M, Ernst S (2010) Production of ethanol and feed by high dry matter hydrolysis and fermentation of palm kernel press cake. Appl Biochem Biotechnol 161:318–332
Katrolia P, Zhou P, Zhang P, Yan Q, Li Y, Jiang Z, Xu H (2012) High level expression of a novel β-mannanase from Chaetomium sp. exhibiting efficient mannan hydrolysis. Carbohy Pol 87:480–490
Kim DY, Ham SJ, Lee HJ, Kim YJ, Shin DH, Rhee YH, Son KH, Park HY (2011a) A highly active endo-1,4-β-mannanase produced by Cellulosimicrobium sp. strain HY-13, a hemicellulolytic bacterium in the gut of Eisenia fetida. Enzyme Microb Technol 48:365–370
Kim DY, Ham SJ, Lee HJ, Cho HY, Kim JH, Kim YJ, Shin DH, Rhee YH, Son KH, Park HY (2011b) Cloning and characterization of a modular GH5 β-1,4-mannanase with high specific activity from the fibrolytic bacterium Cellulosimicrobium sp. strain HY-13. Biores Technol 102:9185–9192. doi:10.1016/j.biortech.2011.06.073
Kote NV, Patil AGG, Mulimani VH (2009) Optimization of the production of thermostable endo-β-1,4 mannanase from a newly isolated Aspergillus niger gr and Aspergillus flavus gr. Appl Biochem Biotechnol 152:213–223
Kumagai Y, Usuki H, Yamamoto Y, Yamasato A, Arima J, Mukaihara T, Hatanaka T (2011) Characterization of calcium ion sensitive region for β-mannanase from Streptomyces thermolilacinus. Biochim Biophys Acta 1814:1127–1133
Leeds AR, Kang SS, Low AG, Sambrook IE (1980) The pig as a model for studies on the mode of action of guar gum in normal and diabetic man. Proc Nutr Soc 39:44
Li Y, Yang P, Meng K, Wang Y, Luo H, Wu N, Fan Y, Yao B (2008) Gene, cloning, expression and characterization of a novel β-mannanase from Bacillus circulans CGMCC 1416. J Microbiol Biotechnol 18:160–166
Lin SS, Dou WF, Xu H, Li HZ, Xu ZH, Ma Y (2007) Optimization of medium composition for the production of alkaline β-mannanase by alkaliphilic Bacillus sp. N16-5 using response surface methodology. Appl Microbiol Biotechnol 75(5):1015–1022
Luo H, Wang Y, Wang H, Yang J, Yang Y, Huang H, Yang P, Bai Y, Shi P, Fan Y, Yao B (2009) A novel highly acidic β-mannanase from the acidophilic fungus Bispora sp. MEY-1: gene cloning and overexpression in Pichia pastoris. Appl Microbiol Biotechnol 82:453–461
Ma Y, Xue Y, Dou Y, Xu Z, Tao W, Zhou P (2004) Characterization and gene cloning of a novel beta-mannanase from alkalophilic Bacillus sp. N16-5. Extremophiles 8:447–454
Mabrouk MEM, Ahwany AMDEI (2008) Production of β-mannanase by Bacillus amyloliquefaciens 10A1 cultured on potato peels. Afri J Biotechnol 7:1123–1128
Manjula S, Shinde M, Lalitha J (2010) Optimization of culture conditions for the production of β-mannanse from an agar utilizing Paenibacillus sp. MSL-9. The Bioscan 5(1):75–79
Matheson NK (1990) Mannose-based polysaccharides. Met in Plant Biochem 2:371–413
Meenakshi, Singh G, Bhalla A, Hoondal GS (2010) Solid state fermentation and characterization of partially purified thermostable mannanase from Bacillus sp. MG-33. Bioresources 5(3):1689–1701
Mohamad SN, Ramanan RN, Mohamad R, Ariff AB (2011) Improved mannan degrading enzymes production by Aspergillus niger through medium optimization. New Biotechnol 28:146–152. doi:10.1016/j.nbt.2010.10.008
Moreira LRS, Filho EXF (2008) An overview of mannan structure and mannan degrading enzyme systems. Appl Microbiol Biotechnol 79(2):165–178
Mou H, Zhou F, Jiang X, Liu Z (2011) Production, purification and properties of β-mannanase from soil bacterium Bacillus circulans M-21. J Food Biochem 35:1451–1460
Mudau MM, Setati ME (2008) Partial purification and characterization of endo-β-1,4 mannanases from Scopularipsis candida strains isolated from solar salterns. Afri J Biotechnol 7(13):2279–2285
Mussini FJ, Coto CA, Goodgame SD, Lu C, Karimi AJ, Lee JH, Waldroup (2011) Effect of β-mannanase on broiler performance and dry mater output using corn-soyabean meal based diets. Int J Pou Sci 10(10):778–781
Nicolas P, Raetz E, Reymond S, Sauvegeat JL (1998) Hydrolysis of the galactomannans of coffee extract with immobilized β-mannanase. Patent US5714183
Norita S, Rosfarizan M, Ariff AB (2010) Evaluation of the activities of concentrated crude mannan-degrading enzymes produced by Aspergillus niger. Mal J Microbiol 6(2):171–180
Nours LK, Anderson L, Stoll D, Stalbrand H, Leggio LL (2005) The structure and characterization of a modular endo-beta-1,4-mannanase from Cellulomonas fimi. Biochemistry 44:12700–12708
Nunes FM, Reis A, Domingues MR, Coimbra MA (2006) Characterization of galactomannan derivatives in roasted coffee beverages. J Agric Food Chem 54(9):3428–3439
Pan X, Zhou J, Tian A, Le K, Yuan H, Xue Y, Ma Y, Lu H (2011) High level expression of a truncated β-mannanase from alkalophilic Bacillus sp. N16-5 in Kluyveromyces cicerisporus. Biotechnol Lett 33:565–570
Parisi GC, Zilli M, Miani MP, Carrara M, Bottona E, Verdianelli G, Battaglia G, Desideri S, Faedo A, Marzolino C, Tonon A, Ermani M, Leandro G (2002) High-fibre diet supplementation in patients with irritable bowel syndrome (IBS): a multicenter, randomized, open trial comparison between wheat bran diet and partially hydrolyzed guar gum (PHGG) Diges Disea Sci 47(8):1697–1704
Pee V, Ignatius KL, Speybroeck V, Michel MP, Jozef VP (2002) Use of mannanases as a slime control agents. Patent EP0871596 Application Number: EP19960916095
Petrus J, Zyl V, Moodely V, Rose SH, Roth RL, Zyl WHV (2009) Production of the Aspergillus aculeatus endo-1,4-β-mannanase in A. niger. J Ind Microbiol Biotechnol 36:611–617
Pham TA, Berrin JG, Record E, To KA, Sigoillot JC (2010) Hydrolysis of softwood by Aspergillus mannanase: role of a carbohydrate-binding module. J Biotechnol 148:163–170
Polizeli MLTM, Rizzatti ACS, Monti R, Terenzi HF, Jorge JA, Amorim DS (2005) Xylanase from fungi: properties and industrial applications. Appl Microbiol Biotechnol 67:577–591
Puls J, Schuseil J (1993) Chemistry of hemicellulose: relationship between hemicellulose structure and enzyme required for hydrolysis. In: Coughlan MP, Hazlewood GP (eds) Hemicellulose and hemicellulases. Portland, London, pp 1–27
Qiao J, Rao Z, Dong B, Cao Y (2010) Expression of Bacillus subtilis MA- 139 beta-mannanase in Pichia pastoris and the enzyme characterization. Appl Biochem Biotechnol 160(5):1362–1370
Rashid SA, Darah I, Omar IC (2010) Utilization of palm kernel cake for the production of mannanase by an indigenous filamentous fungus, Aspergillus niger USM F4 under solid state fermentation. Int Microbiol 9:1
Rattanasuk S, Cairns MK (2009) Chryseobacterium indologenes novel mannanase-producing bacteria. Songklanakarin. J Sci Technol 31(4):395–399
Sakka M, Goto M, Fujino T, Fujino E, Karita S, Kimura T, Sakka K (2010) Analysis of a Clostridium josui cellulose gene cluster containing the man5A gene and characterization of a recombinant Man5A. Biosci Biotechnol Biochem 74(10):1–6
Santos CR, Squina FM, Navarro AM, Ruller R, Prade R, Murakami MT (2010) Cloning, expression, purification, crystallization and preliminary X-ray diffraction studies of the catalytic domain of a hyperthermostable endo-1,4-β-D-mannanase from Thermotoga petrophila RKU-1. Struc Bio Cryst Communi F66:1078–1081
Scheller, Ulvskov (2010) Hemicelluloses. Annu Rev Plant Biol 61:263–289
Schroder R, Atkinson RG, Redgwell RJ (2009) Re-interpreting the role of endo-β-mannanases as mannan endotransglycosylase/hydrolases in the plant cell wall. Ann Bot 104(2):197–204
Shallom D, Shoham Y (2003) Microbial hemicellulases. Curr Opin Microbiol 6:219–228
Shi P, Yuan T, Zhao J, Huang H, Luo H, Meng K, Wang Y, Yao B (2011) Genetic and biochemical characterization of a protease–resistant mesophilic β-mannanase from Streptomyces sp. S27. J Ind Microbiol Biotechnol 38:451–458. doi:10.1007/s10295-010-0789-3
Songsiriritthigul C, Lapboonrueng S, Roytrakul S, Haltrich D, Yamabhai M (2011) Crystallization and preliminary crystallographic analysis of β-mannanase from Bacillus licheniformis. Struc Biol Crystal Commu F67:217–220. doi:10.1107/S1744309110049067
Summpunn P, Chaijan S, Isarangkul D, Wiyakrutta S, Meevootisom V (2011) Characterization, gene cloning and heterologous expression of β-Mannanase from a thermophilic Bacillus subtilis. J Microbiol 49(1):86–93
Sunna A (2010) Modular organization and functional analysis of dissected modular β-mannanase CsMan26 from Caldicellulosiruptor Rt8B.4. Appl Microbiol Biotechnol 86:189–200
Tailford LE, Ducros VMA, Flint JE, Roberts SM, Morland C, Zechel DL, Smith N, Bjornvad ME, Borchert TV, Wilson KS, Davies GJ, Gilbert HJ (2009) Understanding how diverse β-mannanases recognize heterogenous substrates. Biochemistry 48:7009–7018
Takeno F, Yamada H, Sekiya K, Fujitani B, Ohtsu K (1990) Effect of partially decomposed guar gum on high-cholesterol-fed rats and non-dietary fiber-fed rats. J Jpn Soc Nutr Food Sci 43:421–425
Tanaka M, Umemoto Y, Okamura H, Nakano D, Tamaru Y, Arak T (2009) Cloning and characterization of a β-1,4-mannanase 5C possessing a family 27 carbohydraate–binding module from a marine bacterium, Vibrio sp. strain MA-138. Biosci Biotechnol Biochem 73:109–116
Titapoka S, Keawsompong S, Haltrich D, Nitisinprasert S (2008) Selection and characterization of mannanase producing bacteria useful for the formation of pre biotic manno oligosaccharides from copra meal. World J Microbiol Biotechnol 24:1425–1433
Tunnicliffe RB, Bolam DN, Pell G, Gilbert HJ, Williamson MP (2005) Structure of a mannan-specific family 35 carbohydrate binding module: evidence for significant conformational changes upon ligand binding. J Mol Biol 347:287–296
Van Zyl WH, Rose SH, Trollope K, Gorgens JF (2010) Fungal β-mannanases: mannan hydrolysis, heterologous production and biotechnological applications. Pro Biochem 45:203–1213
Varnai A, Huikko L, Pere J, Siika-aho M, Viikari L (2011) Synergistic action of xylanase and mannanase improves the total hydrolysis of softwood. Biores Technol 102:9096–9104
Wang J, Shao Z, Hong Y, Li C, Fu X, Liu Z (2010) A novel β- mannanase from Pantoea agglomerans A021: gene cloning, expression, purification and characterization. World J Microbiol Biotechnol 26:1777–1784. doi:10.1007/s11274-010-0358-y
Withers SG (2001) Mechanisms of glycosyl transferases and hydrolases. Carbohydr Polym 44(4):325–327
Yamabhai M, Emrat S, Sukasem S, Pesatcha P, Jaruseranee N, Buranabanyat B (2008) Secretion of recombinant Bacillus hydrolytic enzymes using Escherichia coli expression systems. J Biotechnol 133:50–57
Yang P, Li Y, Wang Y, Meng K, Luo H, Yuan Y, Bai Y, Zhan Z, Yao B (2009a) A novel β-mannanase with high specific activity from Bacillus circulans CGMCC1554: gene cloning, expression and enzymatic characterization. App Biochem Biotechnol 159:85–94
Yang XS, Jiang ZB, Song HT, Jiang SJ, Madzak C, Ma LX (2009b) Cell surface display of the active mannanase in Yarrowia lipolytica with a novel surface display system. Appl Biochem Biotechnol 54:171–176
Yoon KH, Lim BL (2007) Cloning and strong expression of a Bacillus subtilis WL-3 mannanase gene in Bacillus subtilis. J Microbiol Biotechnol 17:1688–1694
Zhang SF, Song JH, Wu MC, Sheng JP, Li JF (2008) Mutation breeding of Aspergillus niger strain LW-1 for high-yield β-mannanase production. Chin Agri Biotechnol 16(2):346–350
Zhang M, Chen XL, Zhang ZH, Sun CY, Chen LL, He HL, Zhou BC, Zhang YZ (2009) Purification and functional characterization of endo-β-mannanase MAN5 and its application in oligosaccharides production from konjac flour. Appl Microbiol Biotechnol 83:865–873
Zhao Y, Xue Y, Ma Y (2009) Recent advances and prospect on structural biology of beta-mannanase—a review. Wei Sheng Wu Xue Bao 49(9):1131–1137
Zhao J, Shi P, Luo H, Yang P, Zhao H, Bai Y, Huang H, Wang H, Yao B (2010) An acidophilic and acid-stable β-mannanase from Phialophora sp. P13 with high mannan hydrolysis activity under simulated gastric conditions. J Agric Food Chem 58:3184–3190
Zhao Y, Zhang Y, Cao Y, Qi J, Mao L, Xue Y, Gao F, Hao P, Wang X, Gao GF, Ma Y (2011) Structural analysis of alkaline β-mannanase from alkalophilic Bacillus sp. N16-5: implication for adaptation to alkaline conditions. PLoS One 6(1):e14608. doi:10.1371/journal.pone.0014608
Zhou HY, Pan HY, Rao LQ, Wu YY (2011) Redesign the α/β fold to enhance the stability of mannanase Man23 from Bacillus subtilis. Appl Biochem Biotechnol 163:186–194. doi:10.1007/s12010-010-9027-8
Acknowledgments
This work was supported by Department of Biotechnology, Government of India, New Delhi (grant number BT/PR11 384/GBD/27/157/2008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chauhan, P.S., Puri, N., Sharma, P. et al. Mannanases: microbial sources, production, properties and potential biotechnological applications. Appl Microbiol Biotechnol 93, 1817–1830 (2012). https://doi.org/10.1007/s00253-012-3887-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-3887-5