Abstract
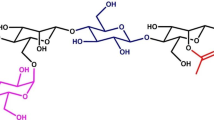
Hemicellulose is a complex group of heterogeneous polymers and represents one of the major sources of renewable organic matter. Mannan is one of the major constituent groups of hemicellulose in the wall of higher plants. It comprises linear or branched polymers derived from sugars such as d-mannose, d-galactose, and d-glucose. The principal component of softwood hemicellulose is glucomannan. Structural studies revealed that the galactosyl side chain hydrogen interacts to the mannan backbone intramolecularly and provides structural stability. Differences in the distribution of d-galactosyl units along the mannan structure are found in galactomannans from different sources. Acetyl groups were identified and distributed irregularly in glucomannan. Some of the mannosyl units of galactoglucomannan are partially substituted by O-acetyl groups. Some unusual structures are found in the mannan family from seaweed, showing a complex system of sulfated structure. Endohydrolases and exohydrolases are involved in the breakdown of the mannan backbone to oligosaccharides or fermentable sugars. The main-chain mannan-degrading enzymes include β-mannanase, β-glucosidase, and β-mannosidase. Additional enzymes such as acetyl mannan esterase and α-galactosidase are required to remove side-chain substituents that are attached at various points on mannan, creating more sites for subsequent enzymatic hydrolysis. Mannan-degrading enzymes have found applications in the pharmaceutical, food, feed, and pulp and paper industries. This review reports the structure of mannans and some biochemical properties and applications of mannan-degrading enzymes.



Similar content being viewed by others
References
Ademark P, Varga A, Medve J, Harjunpää V, Drakenberg T, Tjerneld F, Stålbrand H (1998) Softwood hemicellulose-degrading enzymes from Aspergillus niger: purification and properties of a β-mannanase. J Biotechnol 63:199–210
Ademark P, Larsson M, Tjerneld F, Stålbrand H (2001) Multiple α-galactosidases from Aspergillus niger: purification, characterization and substrate specificities. Enzyme Microb Technol 29:441–448
Andreotti G, Giordano A, Tramice A, Mollo E, Trincone A (2005) Purification and characterization of a β-D-mannosidase from the marine anaspidean Aplysia fasciata. J Biotechnol 119:26–35
Arcand N, Kluepfel D, Paradis FW, Morosoli R, Shareck F (1993) Beta-mannanase of Streptomyces lividans 66: cloning and DNA sequence of the manA gene and characterization of the enzyme. Biochem J 290:857–863
Asenjo JA, Patrick I (1990) Large-scale protein purification. In: Harris ELV, Angal S (eds) Protein purification applications: a practical approach. IRL, Oxford, pp 1–28
Aspinall GO (1959) Structural chemistry of the hemicelluloses. Adv Carbohydr Chem 14:429–468
Aspinall GO, Begbie R, McKay JE (1962) The hemicelluloses of European larch (Larix decidua). Part II. J Chem Soc 214:219
Athanasopoulos VI, Niranjan K, Rastall RA (2005) The production, purification and characterisation of two novel α-D-mannosidases from Aspergillus phoenicis. Carbohydr Res 340:609–617
Bayer EA, Shimon LJ, Shoham Y, Lamed R (1998) Cellulosomes—structure and ultrastructure. J Struct Biol 124:221–234
Benech RO, Li X, Patton D, Powlowski J, Storms R, Bourbonnais R, Paice M, Tsang A (2007) Recombinant expression, characterization, and pulp prebleaching property of a Phanerochaete chrysosporium endo-β-1,4-mannanase. Enzyme Microb Technol 41:740–747
Braithwaite KL, Black GW, Hazlewood GP, Ali BRS, Gilbert HJ (1995) A non-modular endo-b-1,4-mannanase from Pseudomonas fluorescens subspecies cellulosa. Biochem J 305:1005–1010
Brennan CS, Blake DE, Ellis PR, Schofield JD (1996) effects of guar galactomannan on wheat bread microstructure and on the in vitro and in vivo digestibility of starch in bread. J Cereal Sci 24:151–160
Bresolin TM, Sandler PC, Reicher F, Sierakowski MR, Rinaudo M, Ganter JLM (1997) Viscometric studies on xanthan and galactomannan systems. Carbohydr Polym 33:131–138
Broda P (1992) Biotechnology in the degradation and utilization of lignocellulose. Biodegradation 3:219–238
Bulpin PV, Gidley MJ, Jeffcoat R, Underwood DJ (1990) Development of a biotechnological process for the modification of galactomannan polymers with plant α-galactosidase. Carbohydr Polym 12:155–168
Chaikumpollert O, Methacanon P, Suchiva K (2004) Structural elucidation of hemicelluloses from Vetiver grass. Carbohydr Polym 57:191–196
Chandrasekaran R, Radha A, Okuyama K (1998) Morphology of galactomannans: an X-ray structure analysis of guaran. Carbohydr Res 306:243–255
Chen X, Cao Y, Ding Y, Lu W, Li D (2007) Cloning, functional expression and characterization of Aspergillus sulphureus β-mannanase in Pichia pastoris. J Biotechnol 128:452–461
Collins T, Gerday C, Feller G (2005) Xylanases, xylanase families and extremophilic xylanase. FEMS Microbiol Rev 29:3–23
Considine PJ, Coughlan MP (1989) Production of carbohydrate-hydrolysing enzyme blends by solid-state fermentation. In: Coughlan MP (ed) Enzyme systems for lignocellulose degradation. Elsevier, London, pp 273–281
Coughlan MP (1992) Towards an understanding of the mechanism of action of main chain cleaving xylanases. In: Visser J, Beldman G, Kusters-van Someren MA (eds) Xylans and xylanases. Elsevier, Amsterdam, pp 111–139
Coughlan MP, Tuohy MG, Filho EXF, Puls J, Claeyssens M, Vrsanská M, Hughes M (1993) Enzymological aspects of microbial hemicellulases with emphasis on fungal systems. In: Coughlan MP (ed) Hemicellulose and hemicellulases. Portland, London, pp 53–84
Cristofaro E, Mottu F, Wuhrmann JJ (1974) Involvement of the raffinose family of oligosaccharides in flatulence. In: Sipple HL, MCNutt K (eds) Sugars in nutrition. Academic, New York, pp 313–336
Dea ICM, Morrison A (1975) Chemistry and interactions of seed galactomannans. Adv Carbohydr Chem Biochem 31:241–312
Dey PM (1978) Biochemistry of plant galactomannans. Adv Carbohydr Chem Biochem 35:341–376
Duffaud GD, McCutchen CM, Leduc P, Parker KL, Kelly RM (1997) Purification and characterization of extremely thermostable β-mannanase, β-mannosidase, and α-galactosidase from the hyperthermophilic Eubacterium Thermotoga neapolitana 5068. Appl Environ Microbiol 63:169–177
El-Helow ER, Sabry SA, Khattab AA (1997) Production of β-mannanase by B. subtilis from agro-industrial by-products. Antonie van Leeuwenhoek 71:189–193
Erra-Balsells R, Kolender AA, Matulewicz MC, Nonami H, Cerezo AS (2000) Matrix-assisted ultraviolet laser-desorption ionization time-of-flight mass spectrometry of sulfated mannans from the red seaweed Nothogenia fastigiata. Carbohydr Res 329:157–167
Femenia A, Sanchez ES, Simal S, Rosselló C (1999) Compositional features of polysaccharides from Aloe vera (Aloe barbadensis Miller) plant tissues. Carbohydr Polym 39:109–117
Ferreira HM, Filho EXF (2004) Purification and characterization of a β-mannanase from Trichoderma harzianum strain T4. Carbohydr Polym 57:23–29
Filho EXF (1998) Hemicellulases and biotechnology. In: Pandalai SG (ed) Recent research developments in microbiology. Research Signpost, Trivandrum, pp 165–176
Gomes J, Terler K, Kratzer R, Kainz E, Steiner W (2007) Production of thermostable β-mannosidase by a strain of Thermoascus aurantiacus: isolation, partial purification and characterization of the enzyme. Enzyme Microb Technol 40:969–975
Gübitz GM, Hayn M, Sommerauer M, Steiner W (1996) Mannan-degrading enzymes from Sclerotium rolfsii: characterization and synergism of two endo β-mannanases and β-mannosidase. Biores Technol 58:127–135
Heck JX, Soares LHB, Ayub MAZ (2005) Optimization of xylanase and mannanase production by Bacillus circulans strain BL53 on solid-state cultivation. Enzyme Microb Technol 37:417–423
Heidorne FO, Magalhães PO, Ferraz AL, Milagres AMF (2006) Characterization of hemicellulases and cellulases produced by Ceriporiopsis subvermispora grown on wood under biopulping conditions. Enzyme Microb Technol 38:436–442
Hongshu Z, Jinggan Y, Yan Z (2002) The glucomannan from ramie. Carbohydr Polym 47:83–86
Hossain MZ, Abe J-I, Hizukuri S (1996) Multiple forms of β-mannanase from Bacillus sp. KK01. Enzyme Microb Technol 18:95–98
Howard RL, Abotsi E, Jansen van Rensburg EL, Howard S (2003) Lignocellulose biotechnology: issues of bioconversion and enzyme production. Afr J Biotechnol 2:602–619
Ishurd O, Kermagi A, Zgheel F, Flefla M, Elmabruk M, Yalin W, Kennedy JF, Yuanjiang P (2004) Structural aspects of water-soluble galactomannan from the seeds of Retama raetam. Carbohydr Polym 58:41–44
Ishurd O, Kermagi A, Elghazoun M, Kennedy JF (2006) Structural of a glucomannan from Lupinus varius seed. Carbohydr Polym 65:410–413
Jiang Z, Wei Y, Li D, Chai P, Kusakabe I (2006) High-level production, purification and characterization of a thermostable β-mannanase from the newly isolated Bacillus subtilis WY34. Carbohydr Polym 66:88–96
Johnson KG, Ross NW (1990) Enzymic properties of β-mannanase from Polyporus versicolor. Enzyme Microb Technol 12:960–964
Joshi H, Kapoor VP (2003) Cassia grandis Linn. F. seed galactomannan: structural and crystallographical studies. Carbohydr Res 338:1907–1912
Kenne L, Rossel K-G, Svensson S (1975) Studies on the distribution of the O-acetyl groups in pine glucomannan. Carbohydr Res 44:69–76
Kirk O, Borchet TV, Fuglsang CC (2002) Industrial enzyme applications. Curr Opin Biotechnol 13:345–351
Krishna C (1999) Production of bacterial cellulases by solid state bioprocessing of banana wastes. Biores Technol 69:231–239
Kobayashi H, Suzuki H (1972) Studies on the decomposition of raffinose by α-galactosidase of mold. J Ferment Technol 50:625–632
Kurakake M, Sumida T, Masuda D, Oonishi S, Komaki T (2006) Production of Galacto-manno-oligosaccharides from Guar Gum by β-mannanase from Penicillium oxalicum SO. J Agric Food Chem 54:7885–7889
Larsson AM, Anderson L, Xu B, Munõz IG, Usón I, Janson J-C, Stålbrand H, Ståhlberg J (2006) Three-dimensional crystal structure and enzymic characterization of β-mannanase Man5A from blue mussel Mytilus edulis. J Mol Biol 357:1500–1510
Liepman AH, Nairn CJ, Willats WGT, Sørensen I, Roberts AW, Keegstra K (2007) Functional genomic analysis supports conservation of function among cellulose synthase-like A gene family members and suggest diverse roles of mannans in plants. Plant Physiol 143:1881–1893
Lundqvist J, Teleman A, Junel L, Zacchi G, Dahlman O, Tjerneld F, Stålbrand H (2002) Isolation and characterization of galactoglucomannan from spruce (Picea abies). Carbohydr Polym 48:29–39
Lundqvist J, Jacobs A, Palm M, Zacchi G, Dahlman O, Stålbrand H (2003) Characterization of galactoglucomannan extracted from spruce (Picea abies) by heat-fractionation at different conditions. Carbohydr Polym 51:203–211
Maijala P, Raudaskoski M, Viikari L (1995) Hemicellulolytic enzymes in P- and S-strains of Heterobasidion annosum. Microbiol 141:743–750
Martinez ÁT, Speranza M, Ruiz-Dueñas FJ, Ferreira P, Camarero S, Guillén F, Gutiérrez A, del Rio JC (2005) Biodegradation of lignocellulosics: microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int Microbiol 8:195–204
McCleary BV, Matheson NK (1986) Polysaccharides having a β-D-xylan backbone. Adv Carbohydr Chem Biochem 44:158–164
Meier H (1958) On the structure of cell walls and cell wall mannans from ivory nuts and from dates. Biochim Biophys Acta 28:229–240
Meier H, Reid JSG (1982) Reserve Polysaccharides other than starch in higher plants. In: Loewus FA, Tanner W (eds) Encyclopedia of Plant. vol. 13A. Springer, Berlin, pp 418–471
Millane RP, Hendrixson TL (1994) Crystal-structures of mannans and glucomannans. Carbohydr Polym 25:245–251
Montiel MD, Hernández M, Rodriguez J, Arias ME (2002) Evaluation of an endo-β-mannanase produced by Streptomyces ipomoea CECT 3341 for the biobleaching of pine kraft pulps. Appl Microbiol Biotechnol 58:67–72
Mudau MM, Setati ME (2006) Screening and identification of endomannanase-producing microfungi from hypersaline environments. Curr Microbiol 52:477–481
Northcote DH (1972) Chemistry of the plant cell wall. Annu Rev Plant Physiol 23:113–132
Nunes FM, Domingues MR, Comibra MA (2005) Arabinosyl and glucosyl residues as structural features of acetylated galactomannans from green and roasted coffee infusions. Carbohydr Res 340:1689–1698
Olsson L, Christensen TMIE, Hansen KP, Palmqvist EA (2003) Influence of the carbon source on production of cellulases, hemicellulases, and pectinases by Trichoderma reesei Rut C-30. Enzyme Microb Technol 33:612–619
Omarsdottir S, Petersen BO, Barsett H, Paulsen BS, Duus JØ, Olafsdottir ES (2006) Structural characterization of a highly branched galactomannan from the lichen Peltigera canina by methylation analysis and NMR-spectroscopy. Carbohydr Polym 63:54–60
Ootsuka S, Saga N, Suzuki K-I, Inoue A, Ojima T (2006) Isolation and cloning of an endo-β-1,4-mannanase from Pacific abalone Haliotis discus hannai. J Biotechnol 125:269–280
Painter TJ (1983) Structural evolution of glycans in algae. Pure Appl Chem 55:677–694
Parvathy KS, Susheelamma NS, Tharanathan RN, Gaonkar AK (2005) A simple non-aqueous method for carboxmethylation of galactomannans. Carbohydr Polym 62:137–141
Pérez J, Muñoz-Dourado J, de la Rubia T, Martinez J (2002) Biodegradation and biological treatments of cellulose, hemicellulose and lignin: an overview. Int Microbiol 5:53–63
Petkowicz CLO, Reicher F, Chanzy H, Taravel FR, Vuong R (2001) Linear mannan in the endosperm of Schizolobium amazonicum. Carbohydr Polym 44:107–112
Petkowicz CLO, Schaefer S, Reicher F (2007) The mannan from Schizolobium parahybae endosperm is not a reserve polysaccharide. Carbohydr Polym 69:659–664
Polizeli MLT, Rizzati ACS, Monti R, Terenzi HF, Jorge JA, Amorim DS (2005) Xylanases from fungi: properties and industrial applications. Appl Microbiol Biotechnol 67:577–591
Popa VI, Spiridon J (1998) Hemicelluloses: structure and properties. In: Dumitriu S (ed) Polysaccharides: structural diversity and functional versatility. Marcel Dekker, New York, pp 297–311
Preston RD (1968) Plants without cellulose. Sci Am 216:102–108
Preston RD (1979) Polysaccharide conformation and cell wall function. Annu Rev Plant Physiol 30:55–78
Puchart V, Vršanská M, Svoboda P, Pohl J, Ögel ZB, Biely P (2004) Purification and characterization of two forms of endo-β-1,4-mannanase from a thermotolerant fungus, Aspergillus fumigatus IMI 385708 (formerly Thermomyces lanuginosus IMI 158749). Biochim Biophys Acta 1674:239–250
Puls J, Schuseil J (1993) Chemistry of hemicellulose: relationship between hemicellulose structure and enzyme required for hydrolysis. In: Coughlan MP, Hazlewood GP (eds) Hemicellulose and hemicellulases. Portland, London, pp 1–27
Saake B, Clark T, Puls J (1995) Investigations on the reaction mechanism of xylanases and mannanases on sprucewood chemical pulps. Holzforschung 49:60–68
Sá-Pereira P, Paveia H, Costa-Ferreira M, Aires-Barros MR (2003) A new look at xylanases. Mol Biotechnol 24:257–281
Saha BC (2003) Hemicellulose bioconversion. J Ind Microbiol Biotechnol 30:279–291
Saloheimo M, Paloheimo M, Hakola S, Pere J, Swanson B, Nyyssönen E, Bhatia A, Ward M, Pettlilå M (2002) Swollenin, a Trichoderma reesei protein with sequence similarity to the plant expansins, exhibits disruption activity on cellulosic material. Eur J Biochem 269:4202–4211
Setati ME, Ademark P, van Zyl WH, Hahn-Hägerdal B, Stålbrand H (2001) Expression of the Aspergillus aculeatus endo-β-1,4-mannanase encoding gene (man1) in Sacharomyces cerevisiae and characterization of the recombinant enzyme. Protein Exp Purif 21:105–114
Shobha MS, Kumar ABV, Tharanathan RN, Koka R, Gaonkar AK (2005) Modification of guar galactomannan with the aid of Aspergillus niger pectinase. Carbohydr Polym 62:267–273
Singh S, Madlala AM, Prior BA (2003) Thermomyces lanuginosus: properties of strains and their hemicelulases. FEMS Microbiol Rev 27:3–16
Singh V, Malviya T (2006) A non-ionic glucomannan from the seeds of an indigenous medicinal plant: Bryonia lacinosa. Carbohydr Polym 64:481–483
Sittikijyothin W, Torres D, Gonçalves MP (2005) Modelling the rheological behaviour of galactomannan aqueous solutions. Carbohydr Polym 59:339–350
Stålbrand H (1993) Purification and characterization of two β-mannanases from Trichoderma reesei. J Biotechnol 29:229–242
Stålbrand H, Siika-aho M, Tenkanen M, Viikari L (1993) Purification and characterization of two β-mannanases from Trichoderma reesei. J Biotechnol 29:229–242
Stoll D, Boraston A, Stålbrand H, McLean BW, Kilburn DG, Warren AJ (2000) Mannanase Man26A from Cellulomonas fimi has a mannan-binding module. FEMS Microbiol Lett 183:265–269
Techapun C, Poosaran N, Watanabe M, Sasaki K (2003) Thermostable and alkaline-tolerant microbial cellulase-free xylanases produced from agricultural wastes and the properties required for use in pulp bleaching bioprocesses: a review. Process Biochem 38:1327–1340
Timell TE (1965) Wood Hemicelluloses: part II. Carbohydr Chem 20:409–483
Timell TE (1967) Recent progress in the chemistry of wood hemicelluloses. Wood Sci Technol 1:45–70
Tuohy MG, Buckley RJ, Griffin TO, Connelly IC, Shanley NA, Filho EXF, Hughes MM, Grogan P, Coughlan MP (1989) Enzyme production by solid-state cultures of aerobic fungi on lignocellulosic substrates. In: Coughlan MP (ed) Enzyme systems for lignocellulose degradation. Elsevier, London, pp 293–312
Turner P, Mamo G, Karlsson EN (2007) Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb Cell Fact 6:1–23
Viikari L, Tenkanen M, Buchert J, Rattö M, Bailey M, Siika-Aho M, Linko M (1993) Hemicellulases for industrial applications. In: Saddler JN (ed) Bioconversion of forest and agricultural plant residues. CAB International, Wallingford, pp 131–182
Willför S, Sjöholm R, Laine C, Roslund M, Hemming J, Holmbom B (2003) Characterisation of water-soluble galactoglucomannan from Norway spruce wood and thermomechanical pulp. Carbohydr Polym 52:175–187
Wong KKY, Saddler JN (1993) Applications of hemicellulases in the food, feed, and pulp and paper industries. In: Coughlan MP, Hazlewood GP (eds) Hemicellulose and hemicellulases. Portland, London, pp 127–143
Ximenes EA, Chen H, Kataeva IA, Cotta MA, Felix CR, Ljungdahl LG, Li X-L (2005) A mannanase, ManA, of the polycentric anaerobic fungus Orpinomyces sp. strain PC-2 has carbohydrate binding and docking modules.. Can J Microbiol 51:559–568
Xu B, Hägglund P, Stålbrand H, Janson J-C (2002) Endo-β-1,4-mannanases from blue mussel, Mytilus edulis: purification, characterization, and mode of action. J. Biotechnol 92:267–277
Zaldivar J, Nielsen J, Olsson L (2001) Fuel Ethanol production from lignocellulose: a challenge for metabolic engineering and process integration. Appl Microbiol Biotechnol 56:17–34
Acknowledgments
E.X.F.F. and L.R.S.M. acknowledge the receipt of research fellowships from CNPq (Brazil) and FINEP (Brazil), respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moreira, L.R.S., Filho, E.X.F. An overview of mannan structure and mannan-degrading enzyme systems. Appl Microbiol Biotechnol 79, 165–178 (2008). https://doi.org/10.1007/s00253-008-1423-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1423-4