Abstract
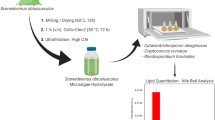
Economic and ecological reasons cause the industry to develop new innovative bio-based processes for the production of oil as renewable feedstock. Petroleum resources are expected to be depleted in the near future. Plant oils as sole substituent are highly criticized because of the competitive utilization of the agricultural area for food and energy feedstock production. Microbial lipids of oleaginous microorganisms are therefore a suitable alternative. To decrease production costs of microbial lipids and gain spatial independence from industrial sites of CO2 emission, a combination of heterotrophic and phototrophic cultivation with integrated CO2 recycling was investigated in this study. A feasibility study on a semi-pilot scale was conducted and showed that the cultivation of the oleaginous yeast Cryptococcus curvatus on a 1.2-L scale was sufficient to supply a culture of the oleaginous microalgae Phaeodactylum tricornutum in a 21-L bubble column reactor with CO2 while single cell oils were produced in both processes due to a nutrient limitation.




Similar content being viewed by others
References
Babel W, Muller RH, Markuske KD (1983) Improvement of growth-yield of yeast on glucose to the maximum by using an additional energy source. Arch Microbiol 136(3):203–208. doi:10.1007/bf00409845
Benemann JR, Tillett DM, Weissman JC (1987) Microalgae biotechnology. Trends Biotechnol 5(2):47–53
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energ Rev 14(2):557–577. doi:10.1016/j.rser.2009.10.009
Brown LM (1996) Uptake of carbon dioxide from flue gas by microalgae. Energy Convers Manag 37(6–8):1363–1367. doi:10.1016/0196-8904(95)00347-9
Camacho Rubio F, Fernández FGA, Pérez JAS, Camacho FG, Grima EM (1999) Prediction of dissolved oxygen and carbon dioxide concentration profiles in tubular photobioreactors for microalgal culture. Biotechnol Bioeng 62(1):71–86. doi:10.1002/(sici)1097-0290(19990105)62:1<71::aid-bit9>3.0.co;2-t
Carlsson AS (2009) Plant oils as feedstock alternatives to petroleum—a short survey of potential oil crop platforms. Biochimie 91(6):665–670. doi:10.1016/j.biochi.2009.03.021
Carmo AC Jr, de Souza LKC, da Costa CEF, Longo E, Zamian JR, da Rocha Filho GN (2009) Production of biodiesel by esterification of palmitic acid over mesoporous aluminosilicate Al-MCM-41. Fuel 88(3):461–468. doi:10.1016/j.fuel.2008.10.007
Cerff M, Morweiser M, Dillschneider R, Michel A, Menzel K, Posten C (2012) Harvesting fresh water and marine algae by magnetic separation: screening of separation parameters and high gradient magnetic filtration. Bioresour Technol 118:289–295
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25(3):294–306. doi:10.1016/j.biotechadv.2007.02.001
Chisti Y, Moo-Young M (1986) Disruption of microbial cells for intracellular products. Enzym Microb Technol 8(4):194–204. doi:10.1016/0141-0229(86)90087-6
Clarens AF, Resurreccion EP, White MA, Colosi LM (2010) Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ Sci Technol 44(5):1813–1819. doi:10.1021/es902838n
Dillschneider R, Steinweg C, Rosello-Sastre R, Posten C (2013) Biofuels from microalgae: photoconversion efficiency during lipid accumulation. Bioresour Technol 142:647–654
Doucha J, Lívanský K (2009) Outdoor open thin-layer microalgal photobioreactor: potential productivity. J Appl Phycol 21(1):111–117. doi:10.1007/s10811-008-9336-2
Fábregas J, Vázquez V, Cabezas B, Otero A (1993) Tris not only controls the pH in microalgal cultures, but also feeds bacteria. J Appl Phycol 5(5):543–545. doi:10.1007/bf02182514
Feron PHM, Hendriks CA (2005) Les différents procédés de capture du CO2 et leurs coûts. Oil Gas Sci Technol 60(3):451–459
Galloway RA, Gauch HG, Soeder CJ (1964) Effects of inhibitory levels of CO2 on Chlorella. Plant Physiol 39 (R8). http://www.nature.com/nature/journal/v206/n4979/abs/206035a0.html
García Sánchez JL, Berenguel M, Rodríguez F, Fernández Sevilla JM, Brindley Alias C, Acién Fernández FG (2003) Minimization of carbon losses in pilot-scale outdoor photobioreactors by model-based predictive control. Biotechnol Bioeng 84(5):533–543. doi:10.1002/bit.10819
Hassan M, Blanc PJ, Pareilleux A, Goma G (1994) Production of single-cell oil from prickly-pear juice fermentation by Cryptococcus curvatus grown in batch culture. World J Microbiol Biotechnol 10(5):534–537
Lee JS, Kim DK, Lee JP, Park SC, Koh JH, Cho HS, Kim SW (2002) Effects of SO2 and NO on growth of Chlorella sp. KR-1. Bioresour Technol 82(1):1–4
Liebert (1987) Final report on the safety assessment of oleic acid, lauric acid, palmitic acid, myristic acid and stearic acid. J Am Coll Toxicol 6(3):321–402
Ma FR, Hanna MA (1999) Biodiesel production: a review. Bioresour Technol 70(1):1–15
Mann JE, Myers J (1968) On pigments, growth and photosynthesis of Phaeodactylum tricornutum. J Phycol 4(4):349–355. doi:10.1111/j.1529-8817.1968.tb04707.x
Matsunaga T, Takeyama H, Miura Y, Yamazaki T, Furuya H, Sode K (1995) Screening of marine cyanobacteria for high palmitoleic acid production. FEMS Microbiol Lett 133(1–2):137–141. doi:10.1016/0378-1097(95)00350-e
Meesters PAEP, Huijberts GNM, Eggink G (1996) High-cell-density cultivation of the lipid accumulating yeast Cryptococcus curvatus using glycerol as a carbon source. Appl Microbiol Biotechnol 45(5):575–579. doi:10.1007/s002530050731
Moon NJ, Hammond EG (1978) Oil production by fermentation of lactose and effect of temperature on fatty acid composition. J Am Oil Chem Soc 55(10):683–688. doi:10.1007/bf02665361
Puanbut M, Leesing R (2012) Integrated cultivation technique for microbial lipid production by photosynthetic microalgae and locally oleaginous yeasts. World Acad Sci Eng Technol 64:975
Ratledge C, Cohen Z (2005) Single Cell Oils. Champaign, Illinois: AOCS Press
Ratledge C, Cohen Z (2008) Microbial and algal oils: do they have a future for biodiesel or as commodity oils? Lipid Technol 20(7):155–160. doi:10.1002/lite.200800044
Richardson JW, Outlaw JL, Allison M (2010) The economics of microalgae oil. AgBioforum 13(2):119–130
Schlagermann P, Göttlicher G, Dillschneider R, Rosello-Sastre R, Posten C (2012) Composition of algal oil and its potential as biofuel. J Combust 2012:14. doi:10.1155/2012/285185
Simmonds C (1919) Alcohol, its production, properties, chemistry and industrial applications
Singh A, Nigam PS, Murphy JD (2011) Renewable fuels from algae: an answer to debatable land based fuels. Bioresour Technol 102(1):10–16. doi:10.1016/j.biortech.2010.06.032
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101(2):87–96. doi:10.1263/jbb.101.87
Tredici MR (2010) Photobiology of microalgae mass cultures: understanding the tools for the next green revolution. Biofuels 1(1):143–162. doi:10.4155/bfs.09.10
Varma A, Buscot F (eds) (2005) Microbial metabolism in soil. In: Microorganisms in soils: roles and genesis and functions, Springer pp 129–133
Wijffels RH, Barbosa MJ (2010) An outlook on microalgal biofuels. Science 329(5993):796–799. doi:10.1126/science.1189003
Yamori Y, Nara Y, Tsubouchi T, Sogawa Y, Ikeda K, Horie R (1986) Dietary prevention of stroke and its mechanisms in stroke-prone spontaneously hypertensive rats—preventive effect of dietary fiber and palmitoleic acid. J Hypertens 4(3):449–452
Yang ZH, Miyahara H, Hatanaka A (2011) Chronic administration of palmitoleic acid reduces insulin resistance and hepatic lipid accumulation in KK-A(y) Mice with genetic type 2 diabetes. Lipids Health Dis 10(8):120. doi:10.1186/1476-511x-10-120
Yin P, Chen L, Wang Z, Qu R, Liu X, Xu Q, Ren S (2009) Biodiesel production from esterification of oleic acid over aminophosphonic acid resin D418. Fuel 102(0):499–505. doi:10.1016/j.fuel.2012.05.027
Yongmanitchai W, Ward OP (1991) Growth of and omega-3 fatty acid production by Phaeodactylum tricornutum under different culture conditions. Appl Environ Microbiol 57(2):419–425
Zhang J, Fang X, Zhu X-L, Li Y, Xu H-P, Zhao B-F, Chen L, Zhang X-D (2011) Microbial lipid production by the oleaginous yeast Cryptococcus curvatus O3 grown in fed-batch culture. Biomass Bioenergy 35(5):1906–1911. doi:10.1016/j.biombioe.2011.01.024
Zhao X, Hu CM, Wu SG, Shen HW, Zhao ZK (2011) Lipid production by Rhodosporidium toruloides Y4 using different substrate feeding strategies. J Ind Microbiol Biotechnol 38(5):627–632. doi:10.1007/s10295-010-0808-4
Acknowledgments
This work was funded by the “Bundesministerium für Wirtschaft und Technologie” within the ERA SME project BiCycle Integrated new concept(s) for the production of Single Cell Oils (SCO’s) on an economic scale in cooperation with the companies Evonik Industries AG, EnBW Energie Baden-Württemberg AG, Phytowelt Green Technology GmbH and B.R.A.I.N AG.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Robert Dillschneider and Ines Schulze contributted equally to this paper.
Rights and permissions
About this article
Cite this article
Dillschneider, R., Schulze, I., Neumann, A. et al. Combination of algae and yeast fermentation for an integrated process to produce single cell oils. Appl Microbiol Biotechnol 98, 7793–7802 (2014). https://doi.org/10.1007/s00253-014-5867-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5867-4