Abstract
Background
Studies have demonstrated the beneficial effect of palmitoleic acid (C16:1 n-7) on reducing muscle insulin resistance and preventing beta-cell apoptosis. However, the effect of palmitoleic acid on diabetes remains to be elucidated. The aim of this study was to examine the antidiabetic effect of palmitoleic acid in KK-Ay mice, a spontaneous model for studies of obese type 2 diabetes with low insulin sensitivity.
Methods
KK-Ay mice were orally administered vehicle, 300 mg/kg of palmitoleic acid, or 300 mg/kg of palmitic acid (C16:0) on a daily basis for 4 weeks.
Results
Palmitoleic acid reduced body weight increase, ameliorated the development of hyperglycemia and hypertriglyceridemia, and improved insulin sensitivity. In addition, hepatic characteristics were significantly affected, as weight of the liver and hepatic triglyceride levels were lower in the palmitoleic acid group when compared to the control (vehicle and palmitic acid groups). Oil red O staining clearly indicated reduced hepatic lipid accumulation in response to palmitoleic acid. Furthermore, palmitoleic acid down-regulated mRNA expressions of proinflammatory adipocytokine genes (TNFα and resistin) in white adipose tissue and lipogenic genes (SREBP-1, FAS, and SCD-1) in liver.
Conclusions
These results suggest that palmitoleic acid improves hyperglycemia and hypertriglyceridemia by increasing insulin sensitivity, in part owing to suppressing proinflammatory gene expressions and improving hepatic lipid metabolism in diabetic mice.
Similar content being viewed by others
Background
Type 2 diabetes mellitus, a worldwide health issue, is a cluster of metabolic diseases characterized by hyperglycemia that result from defects in insulin secretion or/and action [1]. Numerous lines of evidence support the involvement of fatty acids in type 2 diabetes mellitus, and many studies have demonstrated that fatty acids with different degrees of saturation have different effects on insulin sensitivity and glucose/lipid metabolism. Saturated fatty acids are key contributors to insulin resistance, whereas unsaturated fatty acids have beneficial effects and improve diabetes pathologies through multiple mechanisms [2, 3]. Palmitoleic acid (C16:1) is an omega-7 monounsaturated fatty acid that is abundant in plant and marine sources [4–6]. It has been demonstrated that palmitoleic acid prevents beta-cell apoptosis induced by glucose or saturated fatty acids [7, 8], and diets rich in palmitoleic acid improve circulating lipid profile in both animal model [9] and human subjects [10, 11]. Furthermore, a recent report demonstrated that palmitoleic acid functions as an adipose tissue-derived lipid hormone that stimulates muscle insulin action and suppresses hepatosteatosis in mice deficient in fatty acid binding protein [12]. Very little data are available, however, concerning the effects of palmitoleic acid on animal models of diabetes.
KK-Ay mice are widely used as a model for type 2 diabetes [13]. Compared with KK mice, the strain with the Ay allele (KK-Ay) are obese and develop severe, early-onset hyperinsulinemia, hyperglycemia, hypertriglyceridemia, and fatty liver. In the present study, we examined the effect of palmitoleic acid on diabetic risk factors in KK-Ay mice, and further investigated the molecular mechanisms underlying these effects.
Methods
Materials
Palmitic acid (C16:0) and palmitoleic acid (C16:1 n-7) in the form of free fatty acids were purchased from Sigma Chemical Company (St. Louis, U.S.A.) and stored at -20°C until use. Polyglycerol ester was obtained from Mitsubishi-Kagaku Foods Corporation (Tokyo, Japan). The fatty acids were dispersed in 1.5% (w/w) of polyglycerol ester aqueous solution (vehicle) by sonification to a final concentration of 30 mg/mL. In the preliminary test, the mean particle diameter and size distribution of fatty acid solutions were measured using a laser diffraction particle size analyser (LS 13320, Beckman Coulter Ltd, Miami FL, USA). All measurements were repeated twice, and no marked differences in emulsion particle sizes between palmitic acid and palmitoleic acid solution were detected (data not shown).
Animals
Six-week-old male KK-Ay mice (SLC, Shizuoka, Japan) were housed individually in stainless wire mesh cages in a room with a 12-h light/dark cycle and a constant temperature of 24 ± 1°C. Mice had free access to distilled water and laboratory chow (Labo MR Stock, Nosan Corporation, Japan) for 1 week to stabilize metabolic conditions. After the stabilization period, 30 mice were randomly divided into 3 equal groups and orally administered either 1.5% (w/w) of polyglycerol ester (vehicle), 300 mg/kg of palmitic acid solution (palmitic acid group), or 300 mg/kg of palmitoleic acid solution (palmitoleic acid group). These dietary supplements were provided through a stomach tube each morning between 10:00 and 11:00 for 4 weeks. All mice were allowed free access to water and chow during the experimental period. We measured each mouse's food intake (every 3-4 days) and body weight (once per week) throughout the experiment. This study was conducted in compliance with the National Institutes of Health: Guide for the Care and Use of Laboratory Animals, and the Institutional Animal Care and Use Committee of Japan SLC Inc. approved the protocol.
Insulin tolerance test
To evaluate effect of palmitoleic acid on insulin sensitivity, an insulin tolerance test was performed on day 22. Following 5 h of food deprivation, an insulin solution (0.5 U/kg body weigh, Humulin R U-100, Eli Lilly, Japan) was administered subcutaneously. Blood samples were collected immediately preceding insulin administration (0 min), and 30, 60, and 120 min later. Blood samples were centrifuged at 2,000 × g for 15 min, and plasma glucose concentrations were measured using a glucose test kit (Glucose CII-test, Wako Pure Chemical Industries, Ltd., Japan).
Blood analysis
To determine plasma glucose levels, blood samples were taken from the retro-orbital venous plexus of each mouse once per week. Samples were drawn from animals in a non-fasted state, immediately preceding administration of the testing materials. On the final day of the experiment, animals were deprived overnight food. The next morning, mice were anesthetized using diethyl ether, and sacrificed by bleeding from the postcaval vein. The blood was collected, and levels of plasma triglyceride, total cholesterol, and free fatty acids were measured using enzyme assay kits (Triglycerol E-Test, Cholesterol E-Test, and NEFA C-Test reagents, respectively; Wako). Plasma insulin concentrations were determined using reagents from an insulin ELISA kit (Morinaga Institute of Biological Science, Inc., Japan).
Organ measurements
On the final day of the study, vital organs were dissected and weighed after a short wash in cold phosphate-buffered saline (PBS, pH 7.4). Mesenteric adipose tissue and livers were maintained at -80°C until analyzed.
Liver triglyceride contents analysis
Liver total lipids were extracted from a portion of the tissue sample as described by Folch et al. [14]. Extracted lipids were dried using a vacuum concentrator (Concentrator Plus 5305, Eppendorf) and dissolved in 2-propanol containing 10% (w/w) Triton X-100. Triglyceride concentrations were determined using Wako Triglyceride E-Test.
Oil red O staining of liver sections
Liver samples were washed in cold PBS and fixed overnight at 4°C with a 4% paraformaldehyde solution in PBS. Samples were embedded in optimal cutting temperature compound (Tissue-Tek, Sakura Finetek Japan Co., Ltd.) and frozen at -80°C. Six-μm thick sections were cut using a cryotome (Jung Frigocut model 2800N, Leica) at -25°C. Sections were stained for 20 min with fresh oil red O solution (Sigma). Stained liver sections were washed thoroughly with distilled water prior to microscopic observation. Images were captured using an Olympus camera mounted on an Olympus upright microscope.
Real-time polymerase chain reaction (RT-PCR)
Mesenteric adipose tissue and liver total RNA were extracted with TRIzol reagent (Qiagen, Valencia, CA), and cDNAs were synthesized from total RNA (1 μg) by using a PrimeScript 1st-strand cDNA synthesis kit (TaKaRa Bio, Otsu, Japan) following manufacturer's protocols. To quantify mRNA expression levels, the PCR amplifications of different cDNAs were performed with SYBR premix Ex Taq (TaKaRa Bio) on the Applied Biosystems 7300 Real-Time PCR System (Life Technologies Co., Japan) according to the manufacturer's instructions. The expression levels of target gene were normalized by the expression of the endogenous control gene encoding 18S ribosomal RNA. The primer sequences used were shown in Table 1.
Statistical Analysis
Data are expressed as mean ± standard errors (SE). Statistical analyses with Tukey multiple comparisons test were performed to determine differences between groups. The results were considered to be significant if the value of p was < 0.05.
Results
Effect of palmitoleic acid on body and organ weights
As shown in Table 2, administration of palmitoleic acid to KK-Ay mice caused a significant decrease (p < 0.05) in body and liver weights, as well as a marked increase (p < 0.05) in pancreas weight as compared with the palmitic acid or control group. Mesenteric white adipose tissue, brown adipose tissue, and skeletal muscle, however, did not differ significantly among the three groups. In addition, food intake of the palmitoleic acid group was lower than in the control group (p < 0.05), whereas palmitic acid did not affect food intake.
Effect of palmitoleic acid on plasma glucose level
Palmitoleic acid administration significantly decreased (p < 0.05) plasma glucose levels compared to animals administered the vehicle alone (Figure 1). In contrast, plasma glucose concentrations did not differ between the palmitic acid and control groups.
Effect of palmitoleic acid on plasma glucose concentrations in KK-Ay mice. Animals were orally administered vehicle (control), 300 mg/kg of palmitic acid, or 300 mg/kg palmitoleic acid on a daily basis for 4 weeks. Values are means ± SE, n = 10. *p < 0.05 compared with the control group. C16:0, palmitic acid; C16:1, palmitoleic acid.
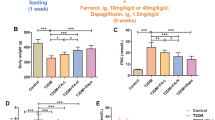
Effect of palmitoleic acid on insulin sensitivity
When animals were subjected to an insulin tolerance test, plasma glucose levels decreased more rapidly in the palmitoleic acid group than in the control group (Figure 2A). In the palmitoleic acid group, plasma glucose concentrations were lower than in the control group at 60 min (p < 0.05) following a single subcutaneous administration of insulin. Plasma glucose levels did not differ between the palmitic acid group and control group. Insulin resistance precedes diabetes onset in KK-Ay mice and typically causes higher plasma insulin levels. As shown in Figure 2B, plasma insulin levels were markedly lower (p < 0.05) in palmitoleic acid than in the palmitic acid or control group.
Effect of palmitoleic acid on insulin resistance in KK-Ay mice. Animals were orally administered vehicle (control), 300 mg/kg of palmitic acid, or 300 mg/kg palmitoleic acid on a daily basis for 4 weeks. (A) Plasma glucose levels in an insulin tolerance test. The insulin tolerance test was carried out on day 22 of the 4-week experiment. Animals were deprived of food for 5 h before administration of the insulin. Blood samples were collected immediately before the insulin injection (0 min) and 30, 60, and 120 min later. Values are means ± SE, n = 10. p < 0.1 and *p < 0.05 compared with the control group. (B) Plasma insulin levels. Blood samples were collected at the end of 4-week experiment after over-night fasting. Means in a row with superscripts without common letter are different, p < 0.05. C16:0, palmitic acid; C16:1, palmitoleic acid.
Effect of palmitoleic acid on plasma lipids
Palmitoleic acid treatment significantly decreased plasma levels of triglyceride (p < 0.05) (Table 2), when compared to the palmitic acid or control group. No changes were detected, however, in plasma levels of total cholesterol or free fatty acid.
Effect of palmitoleic acid on liver lipids
Administration of palmitoleic acid led to a noticeable decrease (p < 0.05) in hepatic triglyceride levels (Figure 3A) compared to either the palmitic acid or control group. Oil red O staining of liver sections revealed neutral lipid content. This analysis indicated that palmitoleic acid reduced hepatic neutral lipid accumulation in KK-Ay mice (Figure 3B III) compared to the control group (Figure 3B I). In contrast, no differences were observed between the control and palmitic acid groups (Figure 3B II).
Effect of palmitoleic acid on hepatic steatosis in KK-Ay mice. Animals were orally administered vehicle (control), 300 mg/kg of palmitic acid, or 300 mg/kg palmitoleic acid on a daily basis for 4 weeks. Graphs depicting neutral lipid accumulation in liver tissue are shown. (A) Hepatic triglyceride concentration. Values are means ± SE, n = 10. Means in a row with superscripts without common letter are different, p < 0.05. C16:0, palmitic acid; C16:1, palmitoleic acid. (B) Oil red O staining of liver sections from control (I), palmitic acid (II), and palmitoleic acid (III) groups of KK-Aymice. Images are shown at 20× magnification. Scale bar = 100 μm.
Effect of palmitoleic acid on mRNA Levels of lipogenic genes in liver
As shown in Figure 4, SREBP-1 (p < 0.05), FAS (p < 0.05), and SCD-1 (p < 0.05) mRNA levels in the palmitoleic acid group were significantly lower than the respective levels in the palmitic acid group or control group. However, there were no changes in these lipogenic gene mRNA expressions between the palmitic acid and control group.
Effect of palmitoleic acid on lipogenic gene expression in liver in KK-Ay mice. Animals were orally administered vehicle (control), 300 mg/kg of palmitic acid, or 300 mg/kg palmitoleic acid on a daily basis. (A) Relative mRNA expression levels of SREBP-1; (B) Relative mRNA expression levels of FAS; (C) Relative mRNA expression levels of SCD-1. Each value is the mean ± SE, n = 10. Means in a row with superscripts without common letter are different, p < 0.05. C16:0, palmitic acid; C16:1, palmitoleic acid; SREBP-1, sterol regulatory element binding protein 1; SCD-1, stearoyl-coenzyme A desaturase 1; FAS, fatty acid synthase.
Effect of palmitoleic acid on adipocytokine mRNA Levels in adipose tissue
We measured mRNA levels of adipocytokines that are reported to influence insulin sensitivity (Figure 5). As compared to the palmitic acid or control group, mRNA levels of TNFα and resistin in mesenteric adipose tissue were markedly suppressed (p < 0.05) by the administration of palmitoleic acid for 4 weeks, though no changes in these data were found between the palmitic acid and control group. On the other hand, adiponectin mRNA expression levels did not differ between the control and palmitic acid and palmitoleic acid groups.
Effect of palmitoleic acid on adipocytokine mRNA levels in adipose tissue in KK-Ay mice. Animals were orally administered vehicle (control), 300 mg/kg of palmitic acid, or 300 mg/kg palmitoleic acid on a daily basis. (A) Relative mRNA expression levels of TNFα; (B) Relative mRNA expression levels of resistin; (C) Relative mRNA expression levels of adiponectin. Each value is the mean ± SE, n = 10. Means in a row with superscripts without common letter are different, p < 0.05. C16:0, palmitic acid; C16:1, palmitoleic acid; TNFα, Tumor necrosis factor alpha.
Discussion
Our results demonstrate that palmitoleic acid positively affects hyperglycemia, hypertriglyceridemia, and insulin resistance in spontaneously diabetic KK-Ay mice. Risk factors for type 2 diabetes mellitus include insulin resistance, hyperglycemia, dyslipidemia, and metabolic syndrome, with insulin resistance being the key underlying metabolic perturbation. Insulin resistance is characterized by reduced responsiveness of target tissues (liver, skeletal muscle, adipocytes) to normal circulating levels of insulin, followed by a progressive decline in insulin secretion from the pancreas [15]. Free fatty acids (saturated fatty acids, in particular) promote insulin resistance and reduce glucose utilization in skeletal muscle [16, 17]. However, there are indications that the monounsaturated palmitoleic acid improves glycemic control and increases glucose transport into skeletal muscle cells. This effect is mediated, at least in part, by upregulation of the activities of the glucose transporters GLUT1 and GLUT4 [18, 19]. Hyperinsulinemic-euglycemic clamp studies have shown that palmitoleic acid, in the form of a triglyceride, potentiates the insulin-signaling pathway in mice [12]. In the present study, administration of palmitoleic acid, but not palmitic acid, improved insulin resistance. It is likely, therefore, that the effects of palmitoleic acid on hyperglycemia and hypertriglyceridemia can be attributed to improved insulin sensitivity.
To investigate the possible mechanisms behind the beneficial effect of palmitoleic acid on insulin sensitivity, we extended our study to examine adipocytokine gene expression. Numerous studies have shown the close relationship between insulin resistance-linked type 2 diabetes and adipocytokines that are mainly derived from adipose tissue [20, 21]. Adiponectin, a key adipocytokine, has been shown to increase insulin sensitivity at least in part through stimulating β-oxidation in skeleton muscle and decreasing hepatic glucose output [22]. However, adiponectin mRNA expression levels did not change by palmitoleic acid administration in the present study, suggesting a minor effect of palmitoleic acid on adiponectin on a gene expression level. On the other hand, repeated administration of palmitoleic acid down-regulated mRNA expressions of TNFα and resistin, the adipocytokines that have been demonstrated to contribute to insulin resistance [23, 24]. It is thereby suggested that the beneficial effect of palmitoleic acid on improvement of insulin resistance may be partially owed to suppressing proinflammatory gene expression. In addition, our results also show that pancreas weight increased by repeated administration of palmitoleic acid. In type 2 diabetes, the pancreas fails to produce enough insulin, and evidence suggests that loss of beta cells contributes to this impairment [25, 26]. Preventing beta-cell apoptosis and promoting its proliferation, therefore, may represent an important mechanism for improving the diabetic condition. In vitro studies have shown that palmitoleic acid prevented the beta cells from high glucose- and palmitic acid-induced impairment of beta-cell proliferation possibly via induction of Bcl-2 [27]. Nevertheless, how palmitoleic acid improves beta-cell function and in turn ameliorates insulin resistance in diabetic model has not been entirely elucidated.
Our present data show that palmitoleic acid suppresses lipid accumulation in the liver. To investigate its mechanism, we assessed liver mRNA levels of genes involved in lipogenesis. Analysis of gene expression with RT-PCR indicated that palmitoleic acid markedly down-regulated mRNA expressions of lipogenic genes such as SREBP-1, FAS and SCD-1. SREBP-1 and its target genes FAS as well as SCD-1 are involved in adipogenesis, and SREBP-1 play a central role in regulating fatty acid metabolism [28]. Suppressing effect of palmitoleic acid on hepatic lipid accumulation is thereby possibly due to inhibiting de novo lipogenesis. Cao et al. [12] demonstrated that fatty acid binding protein-deficient mice have dramatically elevated levels of circulating palmitoleic acid compared to wild-type mice, and that high levels of palmitoleic acid down-regulate genes involved in de novo lipogenesis within the liver. It has been reported that hepatic lipid accumulation closely correlates with obesity, insulin resistance, and type 2 diabetes mellitus [29]. In the liver, insulin suppresses hepatic glucose output by inhibiting gluconeogenesis and stimulating glycogen synthesis [30]. In contrast, hepatic steatosis seems to stimulate gluconeogenesis by activating protein kinase C epsilon type and c-Jun N-terminal kinase 1 [31]. These activated proteins subsequently interfere with tyrosine phosphorylation of insulin receptor substance 1 and 2 and impair the ability of insulin to activate glycogen synthase. We therefore infer that palmitoleic acid improves insulin resistance in diabetic mice, at least in part by decreasing hepatic lipid accumulation.
A growing body of evidence indicates that excess body weight is linked to type 2 diabetes [32, 33]. Administration of palmitoleic acid to KK-Ay mice reduced body weight gain, which may in turn have improved glucose and lipid metabolism. Interestingly, palmitoleic acid treatment resulted in a lower food intake compared with the control group, and studies have shown favorable effects of low calorie intake on obesity-induced metabolic disorders possibly by improving insulin sensitivity [34, 35]. Some unsaturated fatty acids, such as the n-3 polyunsaturated eicosapentaenoic acid and docosahexaenoic acid, as well as some short-chain saturated fatty acids, have been shown to regulate secretion of hunger hormones (e.g., the adipose tissue-derived hormone leptin) and gastrointestinal peptides (e.g., glucagon-like peptide-1, cholecystokinin) [36–39]. On the other hand, levels of hunger hormones correlate with energy intake and glucose metabolism [40–42]. The mechanisms by which palmitoleic acid affects food intake, however, remain unresolved.
Conclusions
In conclusion, oral administration of palmitoleic acid to KK-Ay mice dramatically improved their diabetic condition. Body weight increases, hyperglycemia, and hypertriglyceridemia were all reduced in response to palmitoleic acid. In these mice, down-regulation of proinflammatory gene expressions and decreased hepatic lipid accumulation improved insulin sensitivity, which likely led to many of the beneficial effects documented in this study.
Change history
24 August 2021
A Correction to this paper has been published: https://doi.org/10.1186/s12944-021-01513-w
Abbreviations
- FAS:
-
Fatty acid synthase
- RT-PCR:
-
real-time polymerase chain reaction
- SCD-1:
-
Stearoyl CoA desaturase-1
- SREBP-1:
-
Sterol regulatory element binding protein 1
- TNFα:
-
Tumor necrosis factor alpha.
References
DeFronzo RA, Ferrannini E: Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991, 14: 173-194. 10.2337/diacare.14.3.173
Misra A, Singhal N, Khurana L: Obesity, the metabolic syndrome, and type 2 diabetes in developing countries: role of dietary fats and oils. J Am Coll Nutr. 2010, 29: 289S-301S.
Risérus U, Willett WC, Hu FB: Dietary fats and prevention of type 2 diabetes. Prog Lipid Res. 2009, 48: 44-51. 10.1016/j.plipres.2008.10.002
Yang B, Kallio HP: Fatty acid composition of lipids in sea buckthorn (Hippophaë rhamnoides L.) berries of different origins. J Agric Food Chem. 2001, 49: 1939-1947. 10.1021/jf001059s
Maguire LS, O'Sullivan SM, Galvin K, O'Connor TP, O'Brien NM: Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. Int J Food Sci Nutr. 2004, 5: 171-178.
Ozogul Y, Ozogul F, Cicek E, Polat A, Kuley E: Fat content and fatty acid compositions of 34 marine water fish species from the Mediterranean Sea. Int J Food Sci Nutr. 2008, 29: 1-12.
Morgan NG, Dhayal S: Unsaturated fatty acids as cytoprotective agents in the pancreatic beta-cell. Prostaglandins Leukot Essent Fatty Acids. 2010, 82: 231-236. 10.1016/j.plefa.2010.02.018
Morgan NG, Dhayal S, Diakogiannaki E, Welters HJ: Unsaturated fatty acids as cytoprotective agents in the pancreatic beta-cell. Biochem Soc Trans. 2008, 36: 905-908. 10.1042/BST0360905
Matthan NR, Dillard A, Lecker JL, Ip B, Lichtenstein AH: Effects of dietary palmitoleic acid on plasma lipoprotein profile and aortic cholesterol accumulation are similar to those of other unsaturated fatty acids in the F1B golden Syrian hamster. J Nutr. 2009, 139: 215-221.
Griel AE, Cao Y, Bagshaw DD, Cifelli AM, Holub B, Kris-Etherton PM: A macadamia nut-rich diet reduces total and LDL-cholesterol in mildly hypercholesterolemic men and women. J Nutr. 2008, 138: 761-767.
Garg ML, Blake RJ, Wills RB: Macadamia nut consumption lowers plasma total and LDL cholesterol levels in hypercholesterolemic men. J Nutr. 2003, 133: 1060-1063.
Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS: Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008, 134: 933-944. 10.1016/j.cell.2008.07.048
Fujita T, Sugiyama Y, Taketomi S, Sohda T, Kawamatsu Y, Iwatsuka H, Suzuoki Z: Reduction of insulin resistance in obese and/or diabetic animals by 5-[4-(1-methylcyclohexylmethoxy)benzyl]-thiazolidine-2, 4-dione (ADD-3878, U-63, 287, ciglitazone), a new antidiabetic agent. Diabetes. 1983, 32: 804-810. 10.2337/diabetes.32.9.804
Folch J, Lees M, Stanley GHS: A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1956, 226: 497-509.
Fletcher B, Gulanick M, Lamendola C: Risk factors for type 2 diabetes mellitus. J Cardiovasc Nurs. 2002, 16: 17-23.
Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI: Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996, 97: 2859-2865. 10.1172/JCI118742
Boden G: Effects of free fatty acids (FFA) on glucose metabolism: significance for insulin resistance and type 2 diabetes. Exp Clin Endocrinol Diabetes. 2003, 111: 121-124.
Dimopoulos N, Watson M, Sakamoto K, Hundal HS: Differential effects of palmitate and palmitoleate on insulin action and glucose utilization in rat L6 skeletal muscle cells. Biochem J. 2006, 399: 473-481. 10.1042/BJ20060244
Tsuchiya Y, Hatakeyama H, Emoto N, Wagatsuma F, Matsushita S, Kanzaki M: Palmitate-induced down-regulation of sortilin and impaired GLUT4 trafficking in C2C12 myotubes. J Biol Chem. 2010, 285: 34371-34381. 10.1074/jbc.M110.128520
Fasshauer M, Paschke R: Regulation of adipocytokines and insulin resistance. Diabetologia. 2003, 46: 1594-1603. 10.1007/s00125-003-1228-z
Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B: Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006, 17: 4-12.
Lihn AS, Pedersen SB, Richelsen B: Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 2005, 6: 13-21. 10.1111/j.1467-789X.2005.00159.x
Hotamisligil GS: Mechanisms of TNF-alpha-induced insulin resistance. Exp Clin Endocrinol Diabetes. 1999, 107: 119-125. 10.1055/s-0029-1212086
Barnes KM, Miner JL: Role of resistin in insulin sensitivity in rodents and humans. Curr Protein Rept Sci. 2009, 10: 96-107. 10.2174/138920309787315239.
Donath MY, Halban PA: Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia. 2004, 47: 581-589. 10.1007/s00125-004-1336-4
Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC: Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003, 52: 102-110. 10.2337/diabetes.52.1.102
Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY: Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes. 2003, 52: 726-733. 10.2337/diabetes.52.3.726
Shimano H: Sterol regulatory element-binding proteins (SREBPs): transcriptional regulators of lipid synthetic genes. Prog Lipid Res. 2001, 40: 439-452. 10.1016/S0163-7827(01)00010-8
Lockman KA, Nyirenda MJ: Interrelationships between hepatic fat and insulin resistance in non-alcoholic fatty liver disease. Curr Diabetes Rev. 2010, 6: 341-347. 10.2174/157339910793360879
Taniguchi CM, Emanuelli B, Kahn CR: Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006, 7: 85-96.
Angulo P: Nonalcoholic fatty liver disease. N Engl J Med. 2002, 346: 1221-1231. 10.1056/NEJMra011775
Holbrook TL, Barrett-Connor E, Wingard DL: The association of lifetime weight and weight control patterns with diabetes among men and women in an adult community. Int J Obes. 1989, 13: 723-729.
Aucott LS: Influences of weight loss on long-term diabetes outcomes. Proc Nutr Soc. 2008, 67: 54-59. 10.1017/S0029665108006022
Paulson QX, Hong J, Holcomb VB, Nunez NP: Effects of body weight and alcohol consumption on insulin sensitivity. Nutr J. 2010, 9: 14- 10.1186/1475-2891-9-14
Walford RL, Mock D, Verdery R, MacCallum T: Caloric restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol Biol Sci. 2002, 57: B211-224. 10.1093/gerona/57.6.B211.
Wang H, Storlien LH, Huang XF: Effects of dietary fat types on body fatness, leptin, and ARC leptin receptor, NPY, and AgRP mRNA expression. Am J Phys Med. 2002, 282: E1352-1359.
Hirasawa1 A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G: Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2004, 11: 90-94.
Oh da Y, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM: GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010, 142: 687-698. 10.1016/j.cell.2010.07.041
Feltrin KL, Little TJ, Meyer JH, Horowitz M, Rades T, Wishart J, Feinle-Bisset C: Comparative effects of intraduodenal infusions of lauric and oleic acids on antropyloroduodenal motility, plasma cholecystokinin and peptide YY, appetite, and energy intake in healthy men. Am J Clin Nutr. 2008, 87: 1181-1187.
Wang Y, Asakawa A, Inui A, Kosai K: Leptin gene therapy in the fight against diabetes. Expert Opin Biol Ther. 2010, 10: 1405-1414. 10.1517/14712598.2010.512286
Little TJ, Horowitz M, Feinle-Bisset C: Role of cholecystokinin in appetite control and body weight regulation. Obes Rev. 2005, 6: 297-306. 10.1111/j.1467-789X.2005.00212.x
Ahrén B: Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009, 8: 369-385. 10.1038/nrd2782
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
ZHY participated in the planning, analysis and manuscript preparation. HM participated in the experiment work. AH participated in the planning and organization of the study. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Yang, ZH., Miyahara, H. & Hatanaka, A. Chronic administration of palmitoleic acid reduces insulin resistance and hepatic lipid accumulation in KK-Ay Mice with genetic type 2 diabetes. Lipids Health Dis 10, 120 (2011). https://doi.org/10.1186/1476-511X-10-120
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-511X-10-120