Abstract
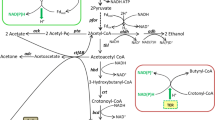
Biosynthesis of acetone and n-butanol is naturally restricted to the group of solventogenic clostridia with Clostridium acetobutylicum being the model organism for acetone-butanol-ethanol (ABE) fermentation. According to limited genetic tools, only a few rational metabolic engineering approaches were conducted in the past to improve the production of butanol, an advanced biofuel. In this study, a phosphotransbutyrylase-(Ptb) negative mutant, C. acetobutylicum ptb::int(87), was generated using the ClosTron methodology for targeted gene knock-out and resulted in a distinct butyrate-negative phenotype. The major end products of fermentation experiments without pH control were acetate (3.2 g/l), lactate (4.0 g/l), and butanol (3.4 g/l). The product pattern of the ptb mutant was altered to high ethanol (12.1 g/l) and butanol (8.0 g/l) titers in pH ≥ 5.0-regulated fermentations. Glucose fed-batch cultivation elevated the ethanol concentration to 32.4 g/l, yielding a more than fourfold increased alcohol to acetone ratio as compared to the wildtype. Although butyrate was never detected in cultures of C. acetobutylicum ptb::int(87), the mutant was still capable to take up butyrate when externally added during the late exponential growth phase. These findings suggest that alternative pathways of butyrate re-assimilation exist in C. acetobutylicum, supposably mediated by acetoacetyl-CoA:acyl-CoA transferase and acetoacetate decarboxylase, as well as reverse reactions of butyrate kinase and Ptb with respect to previous studies.





Similar content being viewed by others
References
Amador-Noguez D, Brasg IA, Feng XJ, Roquet N, Rabinowitz JD (2011) Metabolome remodeling during the acidogenic-solventogenic transition in Clostridium acetobutylicum. Appl Environ Microbiol 77:7984–7997
Andersch W, Bahl H, Gottschalk G (1983) Levels of enzymes involved in acetate, butyrate, acetone and butanol formation by Clostridium acetobutylicum. Eur J Appl Microbiol Biotechnol 18:327–332
Bergmeyer HU (1983) Methods in enzymatic analysis. Verlag Chemie, Weinheim, Germany
Bi C, Jones SW, Hess DR, Tracy BP, Papoutsakis ET (2011) SpoIIE is necessary for asymmetric division, sporulation, and expression of σF, σE, and σG but does not control solvent production in Clostridium acetobutylicum ATCC 824. J Bacteriol 193:5130–5137
Biegel E, Müller V (2010) Bacterial Na+-translocating ferredoxin: NAD+ oxidoreductase. Proc Natl Acad Sci USA 107:18138–18142
Biegel E, Schmidt S, Müller V (2009) Genetic, immunological and biochemical evidence for a Rnf complex in the acetogen Acetobacterium woodii. Environ Microbiol 11:1438–1443
Biegel E, Schmidt S, González JM, Müller V (2011) Biochemistry, evolution and physiological function of the Rnf complex, a novel ion-motive electron transport complex in prokaryotes. Cell Mol Life Sci 68:613–634
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Brooijmans RJW, Poolman B, Schuurman-Wolters GK, De Vos WM, Hugenholtz J (2007) Generation of a membrane potential by Lactococcus lactis through aerobic electron transport. J Bacteriol 189:5203–5209
Brooijmans RJW, De Vos WM, Hugenholtz J (2009) Lactobacillus plantarum WCFS1 electron transport chains. Appl Environ Microbiol 75:3580–3585
Cary JW, Petersen DJ, Papoutsakis ET, Bennett GN (1988) Cloning and expression of Clostridium acetobutylicum phosphotransbutyrylase and butyrate kinase genes in Escherichia coli. J Bacteriol 170:4613–4618
Collins MD, Jones D (1981) Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implications. Microbiol Rev 45:316–354
Das A, Hugenholtz J, Van Halbeek H, Ljungdahl LG (1989) Structure and function of a menaquinone involved in electron transport in membranes of Clostridium thermoautotrophicum and Clostridium thermoaceticum. J Bacteriol 171:5823–5829
Desai RP, Papoutsakis ET (1999) Antisense RNA strategies for metabolic engineering of Clostridium acetobutylicum. Appl Environ Microbiol 65:936–945
Desai RP, Harris LM, Welker NE, Papoutsakis ET (1999) Metabolic flux analysis elucidates the importance of the acid-formation pathways in regulating solvent production by Clostridium acetobutylicum. Metab Eng 1:206–213
Dürre P (2011) Fermentative production of butanol—the academic perspective. Curr Opin Biotechnol 22:331–336
Ezeji T, Milne C, Price ND, Blaschek HP (2010) Achievements and perspectives to overcome the poor solvent resistance in acetone and butanol-producing microorganisms. Appl Microbiol Biotechnol 85:1697–1712
Fontaine L, Meynial-Salles I, Girbal L, Yang X, Croux C, Soucaille P (2002) Molecular characterization and transcriptional analysis of adhE2, the gene encoding the NADH-dependent aldehyde/alcohol dehydrogenase responsible for butanol production in alcohologenic cultures of Clostridium acetobutylicum ATCC 824. J Bacteriol 184:821–830
Gaudu P, Vido K, Cesselin B, Kulakauskas S, Tremblay J, Rezaïki L, Lamberet G, Sourice S, Duwat P, Gruss A (2002) Respiration capacity and consequences in Lactococcus lactis. Antonie Van Leeuwenhoek 82:263–269
Gheshlaghi R, Scharer JM, Moo-Young M, Chou CP (2009) Metabolic pathways of clostridia for producing butanol. Biotechnol Adv 27:764–781
Girbal L, Soucaille P (1998) Regulation of solvent production in Clostridium acetobutylicum. Trends Biotechnol 16:11–16
Girbal L, Vasconcelos I, Soucaille P (1994) Transmembrane pH of Clostridium acetobutylicum is inverted (more acidic inside) when the in vivo activity of hydrogenase is decreased. J Bacteriol 176:6146–6147
Girbal L, Croux C, Vasconcelos I, Soucaille P (1995) Regulation of metabolic shifts in Clostridium acetobutylicum ATCC 824. FEMS Microbiol Rev 17:287–297
Girhard M, Schuster S, Dietrich M, Dürre P, Urlacher VB (2007) Cytochrome P450 monooxygenase from Clostridium acetobutylicum: a new α-fatty acid hydroxylase. Biochem Biophys Res Commun 362:114–119
Gottwald M, Gottschalk G (1985) The internal pH of Clostridium acetobutylicum and its effect on the shift from acid to solvent formation. Arch Microbiol 143:42–46
Gottwald M, Andreesen JR, LeGall J, Ljungdahl LG (1975) Presence of cytochrome and menaquinone in Clostridium formicoaceticum and Clostridium thermoaceticum. J Bacteriol 122:325–328
Grant SG, Jessee J, Bloom FR, Hanahan D (1990) Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc Natl Acad Sci USA 87:4645–4649
Green EM (2011) Fermentative production of butanol—the industrial perspective. Curr Opin Biotechnol 22:337–343
Green EM, Boynton ZL, Harris LM, Rudolph FB, Papoutsakis ET, Bennett GN (1996) Genetic manipulation of acid formation pathways by gene inactivation in Clostridium acetobutylicum ATCC 824. Microbiology 142:2079–2086
Grimmler C, Held C, Liebl W, Ehrenreich A (2010) Transcriptional analysis of catabolite repression in Clostridium acetobutylicum growing on mixtures of d-glucose and d-xylose. J Biotechnol 150:315–323
Grimmler C, Janssen H, Krausse D, Fischer RJ, Bahl H, Dürre P, Liebl W, Ehrenreich A (2011) Genome-wide gene expression analysis of the switch between acidogenesis and solventogenesis in continuous cultures of Clostridium acetobutylicum. J Mol Microbiol Biotechnol 20:1–15
Gu Y, Jiang Y, Wu H, Liu X, Li Z, Li J, Xiao H, Shen Z, Dong H, Yang Y, Li Y, Jiang W, Yang S (2011) Economical challenges to microbial producers of butanol: feedstock, butanol ratio and titer. Biotechnol J 6:1348–1357
Han B, Gopalan V, Ezeji TC (2011) Acetone production in solventogenic Clostridium species: new insights from non-enzymatic decarboxylation of acetoacetate. Appl Microbiol Biotechnol 91:565–576
Harris LM, Desai RP, Welker NE, Papoutsakis ET (2000) Characterization of recombinant strains of the Clostridium acetobutylicum butyrate kinase inactivation mutant: need for new phenomenological models for solventogenesis and butanol inhibition? Biotechnol Bioeng 67:1–11
Hartmanis MGN, Gatenbeck S (1984) Intermediary metabolism in Clostridium acetobutylicum: levels of enzymes involved in the formation of acetate and butyrate. Appl Environ Microbiol 47:1277–1283
Hartmanis GN, Klason T, Gatenbeck S (1984) Uptake and activation of acetate and butyrate in Clostridium acetobutylicum. Appl Microbiol Biotechnol 20:66–71
Heap JT, Pennington OJ, Cartman ST, Carter GP, Minton NP (2007) The ClosTron: a universal gene knock-out system for the genus Clostridium. J Microbiol Methods 70:452–464
Heap JT, Kuehne SA, Ehsaan M, Cartman ST, Cooksley CM, Scott JC, Minton NP (2010) The ClosTron: mutagenesis in Clostridium refined and streamlined. J Microbiol Methods 80:49–55
Herrmann G, Jayamani E, Mai G, Buckel W (2008) Energy conservation via electron-transferring flavoprotein in anaerobic bacteria. J Bacteriol 190:784–791
Hönicke D, Janssen H, Grimmler C, Ehrenreich A, Lütke-Eversloh T (2012) Global transcriptional changes of Clostridium acetobutylicum cultures with increased butanol:acetone ratios. N Biotechnol 29:485–493
Huang L, Gibbins LN, Forsberg CW (1985) Transmembrane pH gradient and membrane potential in Clostridium acetobutylicum during growth under acetogenic and solventogenic conditions. Appl Environ Microbiol 50:1043–1047
Janati-Idrissi R, Junelles AM, El Kanouni A, Petitdemange H, Gay R (1989) Pyruvate fermentation by Clostridium acetobutylicum. Biochem Cell Biol 67:735–739
Janssen H, Döring C, Ehrenreich A, Voigt B, Hecker M, Bahl H, Fischer RJ (2010) A proteomic and transcriptional view of acidogenic and solventogenic steady-state cells of Clostridium acetobutylicum in a chemostat culture. Appl Microbiol Biotechnol 87:2209–2226
Jiang Y, Xu C, Dong F, Yang Y, Jiang W, Yang S (2009) Disruption of the acetoacetate decarboxylase gene in solvent-producing Clostridium acetobutylicum increases the butanol ratio. Metab Eng 11:284–291
Jones DT, Woods DR (1986) Acetone-butanol fermentation revisited. Microbiol Rev 50:484–524
Jones SW, Paredes CJ, Tracy B, Cheng N, Sillers R, Senger RS, Papoutsakis ET (2008) The transcriptional program underlying the physiology of clostridial sporulation. Genome Biol 9:R114
Jones SW, Tracy BP, Gaida SM, Papoutsakis ET (2011) Inactivation of σF in Clostridium acetobutylicum ATCC 824 blocks sporulation prior to asymmetric division and abolishes σE and σG protein expression but does not block solvent formation. J Bacteriol 193:2429–2440
Kuit W, Minton NP, Lopez-Contreras AM, Eggink G (2012) Disruption of the acetate kinase (ack) gene of Clostridium acetobutylicum results in delayed acetate production. Appl Microbiol Biotechnol 94:729–741
Lechardeur D, Cesselin B, Fernandez A, Lamberet G, Garrigues C, Pedersen M, Gaudu P, Gruss A (2011) Using heme as an energy boost for lactic acid bacteria. Curr Opin Biotechnol 22:143–149
Lee SY, Park JH, Jang SH, Nielsen LK, Kim J, Jung KS (2008) Fermentative butanol production by Clostridia. Biotechnol Bioeng 101:209–228
Lee JY, Jang YS, Lee J, Papoutsakis ET, Lee SY (2009) Metabolic engineering of Clostridium acetobutylicum M5 for highly selective butanol production. Biotechnol J 4:1432–1440
Lee J, Jang YS, Choi SJ, Im JA, Song H, Cho JH, Seung DY, Papoutsakis ET, Bennett GN, Lee SY (2012) Metabolic engineering of Clostridium acetobutylicum ATCC 824 for isopropanol-butanol-ethanol fermentation. Appl Environ Microbiol 78:1416–1423
Lehmann D, Lütke-Eversloh T (2011) Switching Clostridium acetobutylicum to an ethanol producer by disruption of the butyrate/butanol fermentative pathway. Metab Eng 13:464–473
Lehmann D, Hönicke D, Ehrenreich A, Schmidt M, Weuster-Botz D, Bahl H, Lütke-Eversloh T (2012) Modifying the product pattern of Clostridium acetobutylicum: physiological effects of disrupting the acetate and acetone formation pathways. Appl Microbiol Biotechnol 94:743–754
Li F, Hinderberger J, Seedorf H, Zhang J, Buckel W, Thauer RK (2008) Coupled ferredoxin and crotonyl coenzyme A (CoA) reduction with NADH catalyzed by the butyryl-CoA dehydrogenase/Etf complex from Clostridium kluyveri. J Bacteriol 190:843–850
Lütke-Eversloh T, Bahl H (2011) Metabolic engineering of Clostridium acetobutylicum: recent advances to improve butanol production. Curr Opin Biotechnol 22:634–647
Lütke-Eversloh T, Fischer A, Remminghorst U, Kawada J, Marchessault RH, Bögershausen A, Kalwei M, Eckert H, Reichelt R, Liu SJ, Steinbüchel A (2002) Biosynthesis of novel thermoplastic polythioesters by engineered Escherichia coli. Nat Mater 1:236–239
Maddox IS, Steiner E, Hirsch S, Wessner S, Gutierrez NA, Gapes JR, Schuster KC (2000) The cause of “acid crash” and “acidogenic fermentations” during the batch acetone-butanol-ethanol (ABE-) fermentation process. J Mol Microbiol Biotechnol 2:95–100
Malca SH, Girhard M, Schuster S, Dürre P, Urlacher VB (2011) Expression, purification and characterization of two Clostridium acetobutylicum flavodoxins: potential electron transfer partners for CYP152A2. Biochim Biophys Acta 1814:257–264
Mermelstein LD, Papoutsakis ET (1993) In vivo methylation in Escherichia coli by the Bacillus subtilis phage phi3T I methyltransferase to protect plasmids from restriction upon transformation of Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol 59:1077–1081
Meyer CL, Roos JW, Papoutsakis ET (1986) Carbon monoxide gasing leads to alcohol production and butyrate uptake without acetone formation in continuous cultures of Clostridium acetobutylicum. Appl Microbiol Biotechnol 24:159–167
Müller V, Imkamp F, Biegel E, Schmidt S, Dilling S (2008) Discovery of a ferredoxin:NAD+-oxidoreductase (Rnf) in Acetobacterium woodii: a novel potential coupling site in acetogens. Ann NY Acad Sci 1125:137–146
Nölling J, Breton G, Omelchenko MV, Makarova KS, Zeng Q, Gibson G, Hong Mei L, Dubois J, Qiu D, Hitti J, Aldredge T, Ayers M, Bashirzadeh R, Bochner H, Boivin M, Bross S, Bush D, Butler C, Caron A, Caruso A, Cook R, Daggett P, Deloughery C, Egan J, Ellston D, Engelstein M, Ezedi J, Gilbert K, Goyal A, Guerin J, Ho T, Holtham K, Joseph P, Keagle P, Kozlovsky J, LaPlante M, LeBlanc G, Lumm W, Majeski A, McDougall S, Mank P, Mao JI, Nocco D, Patwell D, Phillips J, Pothier B, Prabhakar S, Richterich P, Rice P, Rosetti D, Rossetti M, Rubenfield M, Sachdeva M, Snell P, Spadafora R, Spitzer L, Shimer G, Thomann HU, Vicaire R, Wall K, Wang Y, Weinstock K, Lai Peng W, Wonsey A, Xu Q, Zhang L, Wolf YI, Tatusov RL, Sabathe F, Doucette-Stamm L, Soucaille P, Daly MJ, Bennett GN, Koonin EV, Smith DR (2001) Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J Bacteriol 183:4823–4838
Papoutsakis ET (2008) Engineering solventogenic Clostridia. Curr Opin Biotechnol 19:420–429
Roos JW, McLaughlin JK, Papoutsakis ET (1985) The effect of pH on nitrogen supply, cell lysis, and solvent production in fermentations of Clostridium acetobutylicum. Biotechnol Bioeng 27:681–694
Rose IR (1955) Acetate kinase of bacteria (acetokinase). Methods Enzymol 1:591–595
Sambrock J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA
Schmehl M, Jahn A, Meyer zu Vilsendorf A, Hennecke S, Masepohl B, Schuppler M, Marxer M, Oelze J, Klipp W (1993) Identification of a new class of nitrogen fixation genes in Rhodobacter capsulatus: a putative membrane complex involved in electron transport to nitrogenase. Mol Gen Genet 241:602–615
Seedorf H, Fricke WF, Veith B, Brüggemann H, Liesegang H, Strittmatter A, Miethke M, Buckel W, Hinderberger J, Li F, Hagemeier C, Thauer RK, Gottschalk G (2008) The genome of Clostridium kluyveri, a strict anaerobe with unique metabolic features. Proc Natl Acad Sci USA 105:2128–2133
Servinsky MD, Kiel JT, Dupuy NF, Sund CJ (2010) Transcriptional analysis of differential carbohydrate utilization by Clostridium acetobutylicum. Microbiology 156:3478–3491
Sillers R, Al-Hinai MA, Papoutsakis ET (2009) Aldehyde-alcohol dehydrogenase and/or thiolase overexpression coupled with CoA transferase downregulation lead to higher alcohol titers and selectivity in Clostridium acetobutylicum fermentations. Biotechnol Bioeng 102:38–49
Sillers R, Chow A, Tracy B, Papoutsakis ET (2008) Metabolic engineering of the non-sporulating, non-solventogenic Clostridium acetobutylicum strain M5 to produce butanol without acetone demonstrate the robustness of the acid-formation pathways and the importance of the electron balance. Metab Eng 10:321–332
Steiner E, Dago AE, Young DI, Heap JT, Minton NP, Hoch JA, Young M (2011) Multiple orphan histidine kinases interact directly with Spo0A to control the initiation of endospore formation in Clostridium acetobutylicum. Mol Microbiol 80:641–654
Terracciano JS, Kashket ER (1986) Intracellular conditions required for initiation of solvent production by Clostridium acetobutylicum. Appl Environ Microbiol 52:86–91
Thakor N, Lütke-Eversloh T, Steinbüchel A (2005) Application of the BPEC pathway for large-scale biotechnological production of poly(3-mercaptopropionate) by recombinant Escherichia coli, including a novel in situ isolation method. Appl Environ Microbiol 71:835–841
Thormann K, Feustel L, Lorenz K, Nakotte S, Dürre P (2002) Control of butanol formation in Clostridium acetobutylicum by transcriptional activation. J Bacteriol 184:1966–1973
Tracy BP, Jones SW, Papoutsakis ET (2011) Inactivation of σE and σG in Clostridium acetobutylicum illuminates their roles in clostridial-cell-form biogenesis, granulose synthesis, solventogenesis, and spore morphogenesis. J Bacteriol 193:1414–1426
Tummala SB, Welker NE, Papoutsakis ET (2003) Design of antisense RNA constructs for downregulation of the acetone formation pathway of Clostridium acetobutylicum. J Bacteriol 185:1923–1934
Walter KA, Mermelstein LD, Papoutsakis ET (1994) Studies of recombinant Clostridium acetobutylicum with increased dosages of butyrate formation genes. Ann NY Acad Sci 721:69–72
Wang S, Zhang Y, Dong H, Mao S, Zhu Y, Wang R, Luan G, Li Y (2011) Formic acid triggers the “acid crash” of acetone-butanol-ethanol fermentation by Clostridium acetobutylicum. Appl Environ Microbiol 77:1674–1680
Wiesenborn DP, Rudolph FB, Papoutsakis ET (1989a) Phosphotransbutyrylase from Clostridium acetobutylicum ATCC 824 and its role in acidogenesis. Appl Environ Microbiol 55:317–322
Wiesenborn DP, Rudolph FB, Papoutsakis ET (1989b) Coenzyme A transferase from Clostridium acetobutylicum ATCC 824 and its role in the uptake of acids. Appl Environ Microbiol 55:323–329
Yu M, Zhang Y, Tang IC, Yang ST (2011) Metabolic engineering of Clostridium tyrobutyricum for n-butanol production. Metab Eng 13:373–382
Yu M, Du Y, Jiang W, Chang WL, Yang ST, Tang IC (2012) Effects of different replicons in conjugative plasmids on transformation efficiency, plasmid stability, gene expression and n-butanol biosynthesis in Clostridium tyrobutyricum. Appl Microbiol Biotechnol 93:881–889
Zhang Y, Yu M, Yang ST (2012) Effects of ptb knockout on butyric acid fermentation by Clostridium tyrobutyricum. Biotechnol Prog 28:52–59
Zhao Y, Tomas CA, Rudolph FB, Papoutsakis ET, Bennett GN (2005) Intracellular butyryl phosphate and acetyl phosphate concentrations in Clostridium acetobutylicum and their implications for solvent formation. Appl Environ Microbiol 71:530–537
Acknowledgements
The authors thank Nigel P. Minton and John T. Heap, University of Nottingham for kindly providing the ClosTron plasmids. Experimental support by Hella Goschke, Annekatrin Friedrich, and Madlen Schmidt is gratefully acknowledged. This study was in part financially supported by the Süd-Chemie AG, Munich and the German Federal Ministry of Education and Research (Grant no. 0315419A).
Author information
Authors and Affiliations
Corresponding author
Electronic suppplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1,454 kb)
Rights and permissions
About this article
Cite this article
Lehmann, D., Radomski, N. & Lütke-Eversloh, T. New insights into the butyric acid metabolism of Clostridium acetobutylicum . Appl Microbiol Biotechnol 96, 1325–1339 (2012). https://doi.org/10.1007/s00253-012-4109-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4109-x