Abstract
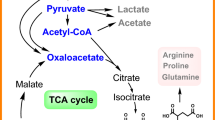
Clostridial acetone–butanol–ethanol (ABE) fermentation is a natural source for microbial n-butanol production and regained much interest in academia and industry in the past years. Due to the difficult genetic accessibility of Clostridium acetobutylicum and other solventogenic clostridia, successful metabolic engineering approaches are still rare. In this study, a set of five knock-out mutants with defects in the central fermentative metabolism were generated using the ClosTron technology, including the construction of targeted double knock-out mutants of C. acetobtuylicum ATCC 824. While disruption of the acetate biosynthetic pathway had no significant impact on the metabolite distribution, mutants with defects in the acetone pathway, including both acetoacetate decarboxylase (Adc)-negative and acetoacetyl-CoA:acyl-CoA transferase (CtfAB)-negative mutants, exhibited high amounts of acetate in the fermentation broth. Distinct butyrate increase and decrease patterns during the course of fermentations provided experimental evidence that butyrate, but not acetate, is re-assimilated via an Adc/CtfAB-independent pathway in C. acetobutylicum. Interestingly, combining the adc and ctfA mutations with a knock-out of the phosphotransacetylase (Pta)-encoding gene, acetate production was drastically reduced, resulting in an increased flux towards butyrate. Except for the Pta-negative single mutant, all mutants exhibited a significantly reduced solvent production.




Similar content being viewed by others
References
Amador-Noguez D, Brasg IA, Feng XJ, Roquet N, Rabinowitz JD (2011) Metabolome remodeling during the acidogenic-solventogenic transition in Clostridium acetobutylicum. Appl Environ Microbiol 77:7984–7997
Atsumi S, Cann AF, Connor MR, Shen CR, Smith KM, Brynildsen MP, Chou KJY, Hanai T, Liao JC (2008) Metabolic engineering of Escherichia coli for 1-butanol production. Metab Eng 10:305–311
Berezina OV, Zakharova NV, Brandt A, Yarotsky SV, Schwarz WH, Zverlov VV (2010) Reconstructing the clostridial n-butanol metabolic pathway in Lactobacillus brevis. Appl Microbiol Biotechnol 87:635–646
Bi C, Jones SW, Hess DR, Tracy BP, Papoutsakis ET (2011) SpoIIE is necessary for asymmetric division, sporulation, and the expression of sF, sE, and sG, but does not control solvent production in Clostridium acetobutylicum. J Bacteriol 193:5130–5137
Bond-Watts BB, Bellerose RJ, Chang MCY (2011) Enzyme mechanism as a kinetic control element for designing synthetic biofuel pathways. Nat Chem Biol 7:222–227
Desai RP, Harris LM, Welker NE, Papoutsakis ET (1999) Metabolic flux analysis elucidates the importance of the acid-formation pathways in regulating solvent production by Clostridium acetobutylicum. Metab Eng 1:206–213
Desai RP, Papoutsakis ET (1999) Antisense RNA strategies for metabolic engineering of Clostridium acetobutylicum. Appl Environ Microbiol 65:936–945
Dong H, Tao W, Zhu L, Zhang Y, Li Y (2011) CAC2634-disrupted mutant of Clostridium acetobutylicum can be electrotransformed in air. Lett Appl Microbiol 53:379–382
Dong H, Zhang Y, Dai Z, Li Y (2010) Engineering Clostridium strain to accept unmethylated DNA. PLoS One 5:e9038
Ezeji T, Milne C, Price ND, Blaschek HP (2010) Achievements and perspectives to overcome the poor solvent resistance in acetone and butanol-producing microorganisms. Appl Microbiol Biotechnol 85:1697–1712
Fischer CR, Tseng HC, Tai M, Prather KLJ, Stephanopoulos G (2010) Assessment of heterologous butyrate and butanol pathway activity by measurement of intracellular pathway intermediates in recombinant Escherichia coli. Appl Microbiol Biotechnol 88:265–275
Gheshlaghi R, Scharer JM, Moo-Young M, Chou CP (2009) Metabolic pathways of clostridia for producing butanol. Biotechnol Adv 27:764–781
Grant SG, Jessee J, Bloom FR, Hanahan D (1990) Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc Natl Acad Sci U S A 87:4645–4649
Green EM (2011) Fermentative production of butanol—the industrial perspective. Curr Opin Biotechnol 22:337–343
Green EM, Boynton ZL, Harris LM, Rudolph FB, Papoutsakis ET, Bennett GN (1996) Genetic manipulation of acid formation pathways by gene inactivation in Clostridium acetobutylicum ATCC 824. Microbiology 142:2079–2086
Grimmler C, Janssen H, Krauße D, Fischer RJ, Bahl H, Dürre P, Liebl W, Ehrenreich A (2011) Genome-wide gene expression analysis of the switch between acidogenesis and solventogenesis in continuous cultures of Clostridium acetobutylicum. J Mol Microbiol Biotechnol 20:1–15
Han B, Gopalan V, Ezeji TC (2011) Acetone production in solventogenic Clostridium species: new insights from non-enzymatic decarboxylation of acetoacetate. Appl Microbiol Biotechnol 91:565–576
Harris LM, Desai RP, Welker NE, Papoutsakis ET (2000) Characterization of recombinant strains of the Clostridium acetobutylicum butyrate kinase inactivation mutant: need for new phenomenological models for solventogenesis and butanol inhibition? Biotechnol Bioeng 67:1–11
Hartmanis MGN (1987) Butyrate kinase from Clostridium acetobutylicum. J Biol Chem 262:617–621
Haus S, Jabbari S, Millat T, Janssen H, Fischer RJ, Bahl H, King JR, Wolkenhauer O (2011) A systems biology approach to investigate the effect of pH-induced gene regulation on solvent production by Clostridium acetobutylicum in continuous culture. BMC Syst Biol 5:10
Heap JT, Kuehne SA, Ehsaan M, Cartman ST, Cooksley CM, Scott JC, Minton NP (2010) The ClosTron: mutagenesis in Clostridium refined and streamlined. J Microbiol Methods 80:49–55
Heap JT, Pennington OJ, Cartman ST, Carter GP, Minton NP (2007) The ClosTron: a universal gene knock-out system for the genus Clostridium. J Microbiol Methods 70:452–464
Inui M, Suda M, Kimura S, Yasuda K, Suzuki H, Toda H, Yamamoto S, Okino S, Suzuki N, Yukawa H (2008) Expression of Clostridium acetobutylicum butanol synthetic genes in Escherichia coli. Appl Microbiol Biotechnol 77:1305–1316
Janssen H, Döring C, Ehrenreich A, Voigt B, Hecker M, Bahl H, Fischer RJ (2010) A proteomic and transcriptional view of acidogenic and solventogenic steady-state cells of Clostridium acetobutylicum in a chemostat culture. Appl Microbiol Biotechnol 87:2209–2226
Jia K, Zhu Y, Zhang Y, Li Y (2011) Group II intron-anchored gene deletion in Clostridium. PLoS One 6:e16693
Jiang Y, Xu C, Dong F, Yang Y, Jiang W, Yang S (2009) Disruption of the acetoacetate decarboxylase gene in solvent-producing Clostridium acetobutylicum increases the butanol ratio. Metab Eng 11:284–291
Jones DT, Woods DR (1986) Acetone–butanol fermentation revisited. Microbiol Rev 50:484–524
Jones SW, Paredes CJ, Tracy B, Cheng N, Sillers R, Senger RS, Papoutsakis ET (2008) The transcriptional program underlying the physiology of clostridial sporulation. Genome Biol 9:R114
Jones SW, Tracy BP, Gaida SM, Papoutsakis ET (2011) Inactivation of sF in Clostridium acetobutylicum ATCC 824 blocks sporulation prior to asymmetric division and abolishes sE and sG protein expression but does not block solvent formation. J Bacteriol 193:2429–2440
Karberg M, Guo H, Zhong J, Coon R, Perutka J, Lambowitz AM (2001) Group II introns as controllable gene targeting vectors for genetic manipulation of bacteria. Nat Biotechnol 19:1162–1167
Lee JY, Jang YS, Lee J, Papoutsakis ET, Lee SY (2009) Metabolic engineering of Clostridium acetobutylicum M5 for highly selective butanol production. Biotechnol J 4:1432–1440
Lee SY, Park JH, Jang SH, Nielsen LK, Kim J, Jung KS (2008) Fermentative butanol production by clostridia. Biotechnol Bioeng 101:209–228
Lehmann D, Lütke-Eversloh T (2011) Switching Clostridium acetobutylicum to an ethanol producer by disruption of the butyrate/butanol fermentative pathway. Metab Eng 13:464–473
Lütke-Eversloh T, Bahl H (2011) Metabolic engineering of Clostridium acetobutylicum: recent advances to improve butanol production. Curr Opin Biotechnol 22:634–647
Mermelstein LD, Papoutsakis ET (1993) In vivo methylation in Escherichia coli by the Bacillus subtilis phage phi3T I methyltransferase to protect plasmids from restriction upon transformation of Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol 59:1077–1081
Monot F, Martin JR, Petitdemange H, Gay R (1982) Acetone and butanol production by Clostridium acetobutylicum in a synthetic medium. Appl Environ Microbiol 44:1318–1324
Nair RV, Papoutsakis ET (1994) Expression of plasmid-encoded aad in Clostridium acetobutylicum M5 restores vigorous butanol production. J Bacteriol 176:5843–5846
Nielsen DR, Leonard E, Yoon SH, Tseng HC, Yuan C, Prather KLJ (2009) Engineering alternative butanol production platforms in heterologous bacteria. Metab Eng 11:262–273
Nölling J, Breton G, Omelchenko MV, Makarova KS, Zeng Q, Gibson G, Hong Mei L, Dubois J, Qiu D, Hitti J, Aldredge T, Ayers M, Bashirzadeh R, Bochner H, Boivin M, Bross S, Bush D, Butler C, Caron A, Caruso A, Cook R, Daggett P, Deloughery C, Egan J, Ellston D, Engelstein M, Ezedi J, Gilbert K, Goyal A, Guerin J, Ho T, Holtham K, Joseph P, Keagle P, Kozlovsky J, LaPlante M, LeBlanc G, Lumm W, Majeski A, McDougall S, Mank P, Mao JI, Nocco D, Patwell D, Phillips J, Pothier B, Prabhakar S, Richterich P, Rice P, Rosetti D, Rossetti M, Rubenfield M, Sachdeva M, Snell P, Spadafora R, Spitzer L, Shimer G, Thomann HU, Vicaire R, Wall K, Wang Y, Weinstock K, Lai Peng W, Wonsey A, Xu Q, Zhang L, Wolf YI, Tatusov RL, Sabathe F, Doucette-Stamm L, Soucaille P, Daly MJ, Bennett GN, Koonin EV, Smith DR (2001) Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J Bacteriol 183:4823–4838
Papoutsakis ET (2008) Engineering solventogenic clostridia. Curr Opin Biotechnol 19:420–429
Paredes CJ, Alsaker KV, Papoutsakis ET (2005) A comparative genomic view of clostridial sporulation and physiology. Nat Rev Microbiol 3:969–978
Ren C, Gu Y, Hu S, Wu Y, Wang P, Yang Y, Yang C, Yang S, Jiang W (2010) Identification and inactivation of pleiotropic regulator CcpA to eliminate glucose repression of xylose utilization in Clostridium acetobutylicum. Metab Eng 12:446–454
Riebe O, Fischer RJ, Wampler DA, Kurtz DM Jr, Bahl H (2009) Pathway for H2O2 and O2 detoxification in Clostridium acetobutylicum. Microbiology 155:16–24
Roos JW, McLaughlin JK, Papoutsakis ET (1985) The effect of pH on nitrogen supply, cell lysis, and solvent production in fermentations of Clostridium acetobutylicum. Biotechnol Bioeng 27:681–694
Rumsby G, Belloque J, Ersser RS, Seakins JW (1987) Effect of temperature and sample preparation on performance of ion-moderated partition chromatography of organic acids in biological fluids. Clin Chim Acta 163:171–183
Sambrock J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Schmidt M, Weuster-Botz D (2012) Reaction engineering studies of acetone-butanol-ethanol fermentation with Clostridium acetobutylicum. Biotechnol J. doi:10.1002/biot.201100418
Shao L, Hu S, Yang Y, Gu Y, Chen J, Jiang W, Yang S (2007) Targeted gene disruption by use of a group II intron (targetron) vector in Clostridium acetobutylicum. Cell Res 17:963–965
Shen CR, Lan EI, Dekishima Y, Baez A, Cho KM, Liao JC (2011) Driving forces enable high-titer anaerobic 1-butanol synthesis in Escherichia coli. Appl Environ Microbiol 77:2905–2915
Sillers R, Al-Hinai MA, Papoutsakis ET (2009) Aldehyde-alcohol dehydrogenase and/or thiolase overexpression coupled with CoA transferase downregulation lead to higher alcohol titers and selectivity in Clostridium acetobutylicum fermentations. Biotechnol Bioeng 102:38–49
Sillers R, Chow A, Tracy B, Papoutsakis ET (2008) Metabolic engineering of the non-sporulating, non-solventogenic Clostridium acetobutylicum strain M5 to produce butanol without acetone demonstrate the robustness of the acid-formation pathways and the importance of the electron balance. Metab Eng 10:321–332
Soucaille P (2008) Process for chromosomal integration and DNA sequence replacement in Clostridia. International patent WO 2008/040387
Steen EJ, Chan R, Prasad N, Myers S, Petzold CJ, Redding A, Ouellet M, Keasling JD (2008) Metabolic engineering of Saccharomyces cerevisiae for the production of n-butanol. Microb Cell Factories 7:36
Steiner E, Dago AE, Young DI, Heap JT, Minton NP, Hoch JA, Young M (2011) Multiple orphan histidine kinases interact directly with Spo0A to control the initiation of endospore formation in Clostridium acetobutylicum. Mol Microbiol 80:641–654
Thormann K, Feustel L, Lorenz K, Nakotte S, Dürre P (2002) Control of butanol formation in Clostridium acetobutylicum by transcriptional activation. J Bacteriol 184:1966–1973
Tracy BP, Jones SW, Papoutsakis ET (2011) Inactivation of sE and sG in Clostridium acetobutylicum illuminates their roles in clostridial-cell-form biogenesis, granulose synthesis, solventogenesis, and spore morphogenesis. J Bacteriol 193:1414–1426
Tummala SB, Junne SG, Papoutsakis ET (2003a) Antisense RNA downregulation of coenzyme A transferase combined with alcohol-aldehyde dehydrogenase overexpression leads to predominantly alcohologenic Clostridium acetobutylicum fermentations. J Bacteriol 185:3644–3653
Tummala SB, Junne SG, Paredes CJ, Papoutsakis ET (2003b) Transcriptional analysis of product-concentration driven changes in cellular programs of recombinant Clostridium acetobutylicum strains. Biotechnol Bioeng 84:842–854
Tummala SB, Welker NE, Papoutsakis ET (2003c) Design of antisense RNA constructs for downregulation of the acetone formation pathway of Clostridium acetobutylicum. J Bacteriol 185:1923–1934
Walter KA, Mermelstein LD, Papoutsakis ET (1994) Studies of recombinant Clostridium acetobutylicum with increased dosages of butyrate formation genes. Ann N Y Acad Sci 721:69–72
Wang S, Zhu Y, Zhang Y, Li Y (2011) Controlling the oxidoreduction potential of the culture of Clostridium acetobutylicum leads to an earlier initiation of solventogenesis, thus increasing solvent productivity. Appl Microbiol Biotechnol. doi:10.1007/s00253-011-3570-2
Wiesenborn DP, Rudolph FB, Papoutsakis ET (1989) Phosphotransbutyrylase from Clostridium acetobutylicum ATCC 824 and its role in acidogenesis. Appl Environ Microbiol 55:317–322
Yu M, Zhang Y, Tang IC, Yang ST (2011) Metabolic engineering of Clostridium tyrobutyricum for n-butanol production. Metab Eng 13:373–382
Xiao H, Gu Y, Ning Y, Yang Y, Mitchell WJ, Jiang W, Yang S (2011) Confirmation and elimination of xylose-metabolic bottlenecks in glucose-PTS-deficient Clostridium acetobutylicum to realize simultaneous utilization of glucose, xylose and arabinose. Appl Environ Microbiol. doi:10.1128/AEM.00644-11
Zhao Y, Tomas CA, Rudolph FB, Papoutsakis ET, Bennett GN (2005) Intracellular butyryl phosphate and acetyl phosphate concentrations in Clostridium acetobutylicum and their implications for solvent formation. Appl Environ Microbiol 71:530–537
Acknowledgements
The authors thank Nigel P. Minton and John T. Heap, University of Nottingham for kindly providing the ClosTron plasmids, and P. Soucaille, Institut nationale des sciences appliquées de Toulouse for kind provision of plasmid pCLF1. Furthermore, experimental support by M. Schmidt and M. Klipp for conducting some of the fermentation experiments is gratefully acknowledged. This study was financially supported by the Süd-Chemie AG, Munich and the German Federal Ministry of Education and Research (grant no. 0315419A).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 744 kb)
Rights and permissions
About this article
Cite this article
Lehmann, D., Hönicke, D., Ehrenreich, A. et al. Modifying the product pattern of Clostridium acetobutylicum . Appl Microbiol Biotechnol 94, 743–754 (2012). https://doi.org/10.1007/s00253-011-3852-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3852-8