Summary
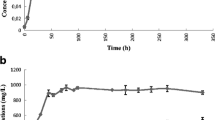
Clostridium acetobutylicum cells were collected from chemostats which were run at pH 4.3 or 6.0 and which produced either acetone-butanol or acetate-butyrate; they were used to determine the level of enzymes involved either in solvent or in acid formation. The highest activity of phosphotransacetylase, phosphotransbutyrylase, acetate kinase, and butyrate kinase was found in cells which carried out an acetate-butyrate fermentation; these enzymes were present in solvent-producing cells at a level of about 10–50% as compared to acid-producing cells. Hydrogenase activity was detectable in approximately the same amounts in both cell types; however, in solvent-producing cells it was only measurable following a lag-period. Butyraldehyde and butanol dehydrogenases were found in small amounts exclusively in solvent-producing cells. It was demonstrated that the formation of acetone was initiated by the action of a coenzyme A-transferase which transferred coenzyme A from acetoacetyl-CoA to either acetate or butyrate. This coenzyme A-transferase as well as acetoacetate decarboxylase were hardly detectable in acid-producing cells, but reached high levels in solvent producing cells. Similar changes of the activity of the enzymes mentioned were observed when a batch culture was shifted from acid to solvent formation.
Similar content being viewed by others
References
Andersch W, Bahl H, Gottschalk G (1982) Acetone-butanol production by Clostridium acetobutylicum in an ammonium-limited chemostat at low pH values. Biotechnol Lett 4:29–32
Bahl H, Andersch W, Braun K, Gottschalk G (1982a) Effect of pH and butyrate concentration on the production of acetone and butanol by Clostridium acetobutylicum grown in continuous culture. Eur J Appl Microbiol Biotechnol 14:17–20
Bahl H, Andersch W, Gottschalk G (1982b) Continuous production of acetone and butanol by Clostridium acetobutylicum in a two-stage phosphate limited chemostat. Eur J Appl Microbiol Biotechnol 15:201–205
Barker HA, Jeng IM, Neff N, Robertson JM, Tam FK, Hosaka S (1978) Butyryl-CoA: acetoacetate CoA-transferase from a lysine-fermenting Clostridium. J Biol Chem 253:1219–1225
Davies R (1943) Studies on the acetone-butanol fermentation. 4. Acetoacetate decarboxylase of C. acetobutylicum (BY). Biochem J 37:230–238
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Gavard R, Hautecoer B, Descourtieux (1957) Phosphotransbutyrylase in Clostridium acetobutylicum. C R Acad Sci [D] (Paris) 244:2323–2326
Hardman JK, Stadtman CT (1963) Metabolism of ω-amino acids. V. Energetics of the γ-aminobutyrate fermentation by Clostridium aminobutyricum. J Bacteriol 85:1326–1333
Lipmann F (1944) Enzymatic synthesis of acyl phosphate. J Biol Chem 155:55–67
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Moreira AR, Dale BE, Doremus MG (1982) Utilisation of the fermentor off-gases from an acetone-butanol fermentation. Biotechnol Bioeng Symp 12:263–277
Petitdemange H, Desborders J, Berthelin J, Gay R (1968) Conversion enzymatique du n-butanol chez Clostridium acetobutylicum. C R Acad Sci [D] (Paris) 266:1772–1774
Petitdemange H, Desborders J, Maugras M (1969) Etude de la formation du n-butanol chez Clostridium acetobutylcium. Bull Soc Chem Biol 51:157–165
Petitdemange H, Cherrier C, Bengone JM, Gay R (1977) Etudes des activites NADH et NADP-ferredoxine oxidoreductasique chez Clostridium acetobutylicum. Can J Microbiol 23:152–160
Simon EJ, Shemin D (1953) The preparation of S-succinyl-CoA J Amer Chem Soc 75:2520
Stadtman ER (1952) The purification and properties of phosphotransacetylase. J Biol Chem 196:527–534
Stadtman ER (1953) The coenzyme A transphorase system in Clostridium kluyveri. J Biol Chem 203:501–512
Stadtman ER, Barker HA (1950) Fatty acid synthesis by enzyme preparations of Clostridium kluyveri. VI. Reactions of acyl phosphates. J Biol Chem 184:769–793
Twarog R, Wolfe RS (1962) Enzymatic phosphorylation of butyrate. J Biol Chem 237:2474–2477
Valentine RC, Wolfe RS (1960) Purification and role of phosphotransbutyrylase. J Biol Chem 235:1948–1952
Zerner B, Coutts SM, Lederer F, Waters HH, Westheimer FH (1966) Acetoacetate decarboxylase. Preparation of the enzyme. Biochemistry 5:813–816
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Andersch, W., Bahl, H. & Gottschalk, G. Level of enzymes involved in acetate, butyrate, acetone and butanol formation by Clostridium acetobutylicum . European J. Appl. Microbiol. Biotechnol. 18, 327–332 (1983). https://doi.org/10.1007/BF00504740
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00504740