Abstract
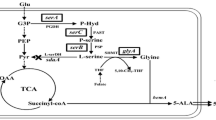
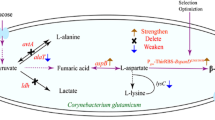
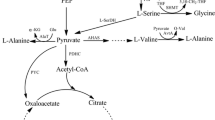
Corynebacterium glutamicum was genetically engineered to produce l-alanine from sugar under oxygen deprivation. The genes associated with production of organic acids in C. glutamicum were inactivated and the alanine dehydrogenase gene (alaD) from Lysinibacillus sphaericus was overexpressed to direct carbon flux from organic acids to alanine. Although the alaD-expressing strain produced alanine from glucose under oxygen deprivation, its productivity was relatively low due to retarded glucose consumption. Homologous overexpression of the gapA gene encoding glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in the alaD-expressing strain stimulated glucose consumption and consequently improved alanine productivity. In contrast gapA overexpression did not affect glucose consumption under aerobic conditions, indicating that oxygen deprivation engendered inefficient regeneration of NAD+ resulting in impaired GAPDH activity and reduced glucose consumption in the alanine-producing strains. Inactivation of the alanine racemase gene allowed production of l-alanine with optical purity greater than 99.5%. The resulting strain produced 98 g l−1 of l-alanine after 32 h in mineral salts medium. Our results show promise for amino acid production under oxygen deprivation.




Similar content being viewed by others
References
Chinen A, Kozlov YI, Hara Y, Izui H, Yasueda H (2007) Innovative metabolic pathway design for efficient L-glutamate production by suppressing CO2 emission. J Biosci Bioeng 103:262–269
Dominguez H, Rollin C, Guyonvarch A, Guerquin-Kern JL, Cocaign-Bousquet M, Lindley ND (1998) Carbon-flux distribution in the central metabolic pathways of Corynebacterium glutamicum during growth on fructose. Eur J Biochem 254:96–102
Ehira S, Shirai T, Teramoto H, Inui M, Yukawa H (2008) Group 2 sigma factor SigB of Corynebacterium glutamicum positively regulates glucose metabolism under conditions of oxygen deprivation. Appl Environ Microbiol 74:5146–5152
Farrell AE, Plevin RJ, Turner BT, Jones AD, O'Hare M, Kammen DM (2006) Ethanol can contribute to energy and environmental goals. Science 311:506–508
Hacking AJ (1986) Economic aspects of biotechnology. Cambridge University Press
Hols P, Kleerebezem M, Schanck AN, Ferain T, Hugenholtz J, Delcour J, de Vos WM (1999) Conversion of Lactococcus lactis from homolactic to homoalanine fermentation through metabolic engineering. Nat Biotechnol 17:588–592
Ikeda M (2003) Amino acid production processes. Adv Biochem Eng Biotechnol 79:1–35
Inui M, Kawaguchi H, Murakami S, Vertès AA, Yukawa H (2004a) Metabolic engineering of Corynebacterium glutamicum for fuel ethanol production under oxygen-deprivation conditions. J Mol Microbiol Biotechnol 8:243–254
Inui M, Murakami S, Okino S, Kawaguchi H, Vertès AA, Yukawa H (2004b) Metabolic analysis of Corynebacterium glutamicum during lactate and succinate productions under oxygen deprivation conditions. J Mol Microbiol Biotechnol 7:182–196
Inui M, Suda M, Okino S, Nonaka H, Puskas LG, Vertès AA, Yukawa H (2007) Transcriptional profiling of Corynebacterium glutamicum metabolism during organic acid production under oxygen deprivation conditions. Microbiology 153:2491–2504
Kawaguchi H, Vertès AA, Okino S, Inui M, Yukawa H (2006) Engineering of a xylose metabolic pathway in Corynebacterium glutamicum. Appl Environ Microbiol 72:3418–3428
Kawaguchi H, Sasaki M, Vertès AA, Inui M, Yukawa H (2008) Engineering of an l-arabinose metabolic pathway in Corynebacterium glutamicum. Appl Microbiol Biotechnol 77:1053–1062
Kinoshita S (1985) Glutamic acid bacteria. In: Demain AL, Solomon NA (eds) Biology of industrial microorganisms. Benjamin Cumings, London, United Kingdom, pp 115–146
Marienhagen J, Kennerknecht N, Sahm H, Eggeling L (2005) Functional analysis of all aminotransferase proteins inferred from the genome sequence of Corynebacterium glutamicum. J Bacteriol 187:7639–7646
Nakata K, Inui M, Kos P, Vertès AA, Yukawa H (2003) Vectors for genetic engineering of Corynebacteria. In: Saha B (ed) American chemical society. vol. symposium series 862: fermentation biotechnology. American Chemical Society, Washington, pp 175–191
Okino S, Inui M, Yukawa H (2005) Production of organic acids by Corynebacterium glutamicum under oxygen deprivation. Appl Microbiol Biotechnol 68:475–480
Okino S, Noburyu R, Suda M, Jojima T, Inui M, Yukawa H (2008a) An efficient succinic acid production process in a metabolically engineered Corynebacterium glutamicum strain. Appl Microbiol Biotechnol 81:459–464
Okino S, Suda M, Fujikura K, Inui M, Yukawa H (2008b) Production of d-lactic acid by Corynebacterium glutamicum under oxygen deprivation. Appl Microbiol Biotechnol 78:449–454
Omumasaba CA, Okai N, Inui M, Yukawa H (2004) Corynebacterium glutamicum glyceraldehyde-3-phosphate dehydrogenase isoforms with opposite, ATP-dependent regulation. J Mol Microbiol Biotechnol 8:91–103
Sasaki M, Jojima T, Inui M, Yukawa H (2008) Simultaneous utilization of d-cellobiose, d-glucose, and d-xylose by recombinant Corynebacterium glutamicum under oxygen-deprived conditions. Appl Microbiol Biotechnol 81:691–699
Sasaki M, Jojima T, Kawaguchi H, Inui M, Yukawa H (2009) Engineering of pentose transport in Corynebacterium glutamicum to improve simultaneous utilization of mixed sugars. Appl Microbiol Biotechnol 85:105–115
Smith GM, Lee SA, Reilly KC, Eiteman MA, Altman E (2006) Fed-batch two-phase production of alanine by a metabolically engineered Escherichia coli. Biotechnol Lett 28:1695–1700
Solem C, Koebmann BJ, Jensen PR (2003) Glyceraldehyde 3-phosphate dehydrogenase has no control over glycolytic flux in Lactococcus lactis MG1363. J Bacteriol 185:1564–1571
Takors R, Bathe B, Rieping M, Hans S, Kelle R, Huthmacher K (2007) Systems biology for industrial strains and fermentation processes—example: amino acids. J Biotechnol 129:181–190
Terasawa M, Yukawa H (1993) Industrial production of biochemicals by native immobilization. In: Tanaka A, Tosa T, Kobayashi T (eds) Industrial application of immobilized biocatalysts. Marcel Dekker, Inc., New York, pp 37–52
Uhlenbusch I, Sahm H, Sprenger GA (1991) Expression of an L-alanine dehydrogenase gene in Zymomonas mobilis and excretion of L-alanine. Appl Environ Microbiol 57:1360–1366
Vertès AA, Inui M, Kobayashi M, Kurusu Y, Yukawa H (1993) Presence of mrr- and mcr-like restriction systems in coryneform bacteria. Res Microbiol 144:181–185
Yasuda K, Jojima T, Suda M, Okino S, Inui M, Yukawa H (2007) Analyses of the acetate-producing pathways in Corynebacterium glutamicum under oxygen-deprived conditions. Appl Microbiol Biotechnol 77:853–860
Yasueda H (2008) The latest development in amino acid fermentation processes. Seibutsu-Kogaku Kaishi 86:525–527
Yukawa H, Omumasaba CA, Nonaka H, Kos P, Okai N, Suzuki N, Suda M, Tsuge Y, Watanabe J, Ikeda Y, Vertès AA, Inui M (2007) Comparative analysis of the Corynebacterium glutamicum group and complete genome sequence of strain R. Microbiology 153:1042–1058
Zhang X, Jantama K, Moore JC, Shanmugam KT, Ingram LO (2007) Production of l-alanine by metabolically engineered Escherichia coli. Appl Microbiol Biotechnol 77:355–366
Acknowledgments
We thank Crispinus A. Omumasaba (RITE) for the critical reading of the manuscript. This work was partially supported by a grant from the New Energy and Industrial Technology Development Organization (NEDO), Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jojima, T., Fujii, M., Mori, E. et al. Engineering of sugar metabolism of Corynebacterium glutamicum for production of amino acid l-alanine under oxygen deprivation. Appl Microbiol Biotechnol 87, 159–165 (2010). https://doi.org/10.1007/s00253-010-2493-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2493-7