Abstract
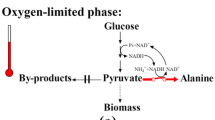
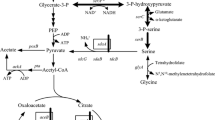
Escherichia coli W was genetically engineered to produce l-alanine as the primary fermentation product from sugars by replacing the native d-lactate dehydrogenase of E. coli SZ194 with alanine dehydrogenase from Geobacillus stearothermophilus. As a result, the heterologous alanine dehydrogenase gene was integrated under the regulation of the native d-lactate dehydrogenase (ldhA) promoter. This homologous promoter is growth-regulated and provides high levels of expression during anaerobic fermentation. Strain XZ111 accumulated alanine as the primary product during glucose fermentation. The methylglyoxal synthase gene (mgsA) was deleted to eliminate low levels of lactate and improve growth, and the catabolic alanine racemase gene (dadX) was deleted to minimize conversion of l-alanine to d-alanine. In these strains, reduced nicotinamide adenine dinucleotide oxidation during alanine biosynthesis is obligately linked to adenosine triphosphate production and cell growth. This linkage provided a basis for metabolic evolution where selection for improvements in growth coselected for increased glycolytic flux and alanine production. The resulting strain, XZ132, produced 1,279 mmol alanine from 120 g l−1 glucose within 48 h during batch fermentation in the mineral salts medium. The alanine yield was 95% on a weight basis (g g−1 glucose) with a chiral purity greater than 99.5% l-alanine.



Similar content being viewed by others
References
Burchhardt G, Ingram LO (1992) Conversion of xylan to ethanol by ethanologenic strains of Escherichia coli and Klebsiella oxytoca. Appl Environ Microbiol 58:1128–1133
Causey TB, Shanmugam KT, Yomano LP, Ingram LO (2004) Engineering Escherichia coli for efficient conversion of glucose to pyruvate. Proc Natl Acad Sci USA 101:2235–2240
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97:6640–6645
Denu JM, Fitzpatrick PF (1992) pH and kinetic isotope effects on the reductive half-reaction of D-amino acid oxidase. Biochemistry 31:8207–8215
Grabar TB, Zhou S, Shanmugam KT, Yomano LP, Ingram LO (2006) Methylglyoxal bypass identified as source of chiral contamination in L(+) and D(−)-lactate fermentations by recombinant Escherichia coli. Biotechnol Lett 28:1527–1535
Hashimoto S, Katsumata R (1993) Overproduction of alanine by Arthrobacter strains with glucose-nonrepressbile L-alanine dehydrogenase. Biotechnol Lett 15:1117–1122
Hashimoto S, Katsumata R (1998) L-alanine fermentation by an alanine racemase-deficient mutant of the DL-alanine hyperproducing bacterium Arthrobacter oxydans HAP-1. J Ferment Bioeng 86:385–390
Hashimoto S, Katsumata R (1999) Mechanism of alanine hyperproduction by arthrobacter oxydans HAP-1: metabolic shift to fermentation under nongrowth aerobic conditions. Appl Environ Microbiol 65:2781–2783
Hols P, Kleerebezem M, Schanck AN, Ferain T, Hugenholtz J, Delcour J, de Vos WM (1999) Conversion of Lactococcus lactis from homolactic to homoalanine fermentation through metabolic engineering. Nat Biotechnol 17:588–592
Ikeda M (2003) Amino acid production processes. Adv Biochem Eng Biotechnol 79:1–35
Karp PD, Ouzounis CA, Moore-Kochlacs C, Goldovsky L, Kaipa P, Ahren D, Tsoka S, Darzentas N, Kunin V, Lopez-Bigas N (2005) Expansion of the BioCyc collection of pathway/genome databases to 160 genomes. Nucleic Acids Res 19:6083–6089
Katsumata R, Hashimoto S (1996) Process for producing alanine. US Patent 5559016
Kuroda S, Tanizawa K, Sakamoto Y, Tanaka H, Soda K (1990) Alanine dehydrogenases from two Bacillus species with distinct thermostabilities: molecular cloning, DNA and protein sequence determination, and structural comparison with other NAD(P)(+)-dependent dehydrogenases. Biochemistry 29:1009–1015
Lai X, Ingram LO (1993) Cloning and sequencing of a cellobiose phosphotransferase system operon from Bacillus stearothermophilus XL-65-6 and functional expression in Escherichia coli. J Bacteriol 175:6441–6450
Lai X, Ingram LO (1995) Discovery of a ptsHI operon, which includes a third gene (ptsT), in the thermophile Bacillus stearothermophilus. Microbiology 141:1443–1449
Lee EC, Yu DG, de Velasco JM, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG (2001) A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics 73:56–65
Lee M, Smith GM, Eiteman MA, Altman E (2004) Aerobic production of alanine by Escherichia coli aceF ldhA mutants expressing the Bacillus sphaericus alaD gene. Appl Microbiol Biotechnol 65:56–60
Lobocka M, Hennig J, Wild J, Klopotowski T (1994) Organization and expression of the Escherichia coli K-12 DAD operon encoding the smaller subunit of D-amino acid dehydrogenase and the catabolic alanine racemase. J Bacteriol 176:1500–1510
Martinez A, Grabar TB, Shanmugam KT, Yomano LP, York SW, Ingram LO (2007) Low salt medium for lactate and ethanol production by recombinant Escherichia coli B. Biotechnol Lett 29:397–404
Martinez-Morales F, Borges AC, Martinez A, Shanmugam KT, Ingram LO (1999) Chromosomal integration of heterologous DNA in Escherichia coli with precise removal of markers and replicons used during construction. J Bacteriol 181:7143–7148
Neidhardt F, Curtiss R III, Ingraham JL, Lin ECC, Low KB Jr, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (1996) Escherichia coli and Salmonella, cellular and molecular biology, 2nd edn. ASM, Washington, DC
Ohashima T, Soda K (1979) Purification and properties of alanine dehydrogenase from Bacillus sphaericus. Eur J Biochem 100:29–30
Orlygsson J, Anderson R, Svensson BH (1995) Alanine as an end product during fermentation of monosaccharides by Clostridium strain P2. Antonie Van Leeuwenhoek 68:273–280
Posfai G, Koob MD, Kirkpatrick HA, Blattner FR (1997) Versatile insertion plasmids for targeted genome manipulations in bacteria: isolation, deletion, and rescue of the pathogenicity island LEE of the Escherichia coli O157:H7 genome. J Bacteriol 179:4426–4428
Shibatani T, Kakimoto T, Chibata I (1979) Stimulation of L-asparate beta-decarboxylase formation by L-glutamate in Pseudomonas dacunhae and improved production of L-alanine. Appl Environ Microbiol 38:359–364
Smith GM, Lee SA, Reilly KC, Eiteman MA, Altman E (2006) Fed-batch two-phase production of alanine by a metabolically engineered Escherichia coli. Biotechnol Lett 28:1695–1700
Thomason L, Court DL, Bubunenko M, Constantino N, Wilson H, Datta S, Oppenheim A (2005) Recombineering: genetic engineering in bacteria using homologous recombination. In: Ausubel FM, Brent R, Klingston RE, Moore DD, Deidman JG, Smith JA, Struhl K (eds) Current protocols in molecular biology. Wiley, New York, p 1.16.1–1.16.21
Totemeyer S, Booth NA, Nichols WW, Dunbar B, Booth IR (1998) From famine to feast: the role of methylglyoxal production in Escherichia coli. Mol Microbiol 27:553–562
Uhlenbusch I, Sahm H, Sprenger GA (1991) Expression of an L-alanine dehydrogenase gene in Zymomonas mobilis and excretion of L-alanine. Appl Environ Microbiol 57:1360–1366
Underwood SA, Buszko ML, Shanmugam KT, Ingram LO (2002) Flux through citrate synthase limits the growth of ethanologenic Escherichia coli KO11 during xylose fermentation. Appl Environ Microbiol 68:1071–1081
Weber J, Kayser A, Rinas U (2005) Metabolic flux analysis of Escherichia coli in glucose-limited continuous culture. II. Dynamic response to famine and feast, activation of the methylglyoxal pathway and oscillatory behaviour. Microbiology 151:707–716
Wild J, Hennig J, Lobocka M, Walczak W, Klopotowski T (1985) Identification of the dadX gene coding for the predominant isozyme of alanine racemase in Escherichia coli K12. Mol Gen Genet 198:315–322
Wood BE, Yomano LP, York SW, Ingram LO (2005) Development of industrial-medium-required elimination of the 2,3-butanediol fermentation pathway to maintain ethanol yield in an ethanologenic strain of Klebsiella oxytoca. Biotechnol Prog 21:1366–1372
Zhou S, Causey TB, Hasona A, Shanmugam KT, Ingram LO (2003a) Production of optically pure D-lactic acid in mineral salts medium by metabolically engineered Escherichia coli W3110. Appl Environ Microbiol 69:399–407
Zhou S, Shanmugam KT, Ingram LO (2003b) Functional replacement of the Escherichia coli D-(−)-lactate dehydrogenase gene (ldhA) with the L-(+)-lactate dehydrogenase gene (ldhL) from Pediococcus acidilactici. Appl Environ Microbiol 69:2237–2244
Zhou S, Grabar TB, Shanmugam KT, Ingram LO (2006a) Betaine tripled the volumetric productivity of D (−)-lactate by Escherichia coli strain SZ132 in mineral salts medium. Biotechnol Lett 28:671–676
Zhou S, Shanmugam KT, Yomano LP, Grabar TB, Ingram LO (2006b) Fermentation of 12% (w/v) glucose to 1.2 M lactate by Escherichia coli strain SZ194 using mineral salts medium. Biotechnol Lett 28:663–670
Acknowledgments
The authors thank Lorraine Yomano and Sean York for their instruction in molecular cloning and fermentation and the University of Florida Interdisciplinary Center for Biotechnology Research (ICBR) for sequencing and amino acid analysis. This research was supported by grants from the US Department of Energy (FG02-96ER20222 and FG36-04GO14019) and BioEnergy International, LLC.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
S1
Metabolic evolution of XZ112 to select XZ113. Strain XZ113 was isolated after 12 serial transfers at 24-h intervals in NBS mineral salts medium containing 50 g l−1 glucose and 1 mM betaine (inoculum of 0.017 mg CDW l−1; pH controlled with 5 N ammonium hydroxide). Fermentation broths were analyzed daily for 3 days. A Cell mass (g l−1). B Alanine production (3 days) (DOC 33 kb)
S2
Effect of pH on cell growth and alanine production by strain XZ123 (NBS mineral salts medium containing 80 g l−1 glucose and 1 mM betaine; inoculum of 0.017 mg CDW l−1). Broth pH was automatically controlled by the addition of 5 N ammonium hydroxide. A Cell mass. B Alanine production. Symbols: □, pH 6.5; △, pH 7.0; ▽, pH 7.5; and ◇, pH 8.0 (DOC 32 kb)
Rights and permissions
About this article
Cite this article
Zhang, X., Jantama, K., Moore, J.C. et al. Production of l-alanine by metabolically engineered Escherichia coli . Appl Microbiol Biotechnol 77, 355–366 (2007). https://doi.org/10.1007/s00253-007-1170-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-1170-y