Abstract
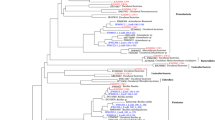
The phylogenetic diversity of the bacterial and archaeal community in the water and sediments of three large lakes of the Wadi An Natrun was investigated using 16S rRNA clone libraries. The bacterial community was diverse: 769 clones formed 345 operational taxonomic units (OTUs) defined at 99% 16S rRNA sequence identity. The bacterial community in both the water and sediments of the lakes was dominated by clones affiliated with the low G + C Gram-type-positive group, α-proteobacteria, and Bacteroidetes, (11–39, 11–30, and 10–37% of OTUs observed, respectively), patterns that have been observed in previously described alkaline, athalassohaline systems. However, a relatively high proportion of Firmicutess-related clones in the water of the lakes and α-proteobacteria in the sediments was observed. The bacterial community composition of the water and sediment of the same lake and of different lakes was significantly different (p < 0.05). Operational taxonomic units related to the γ-proteobacteria were more abundant in the sediment of Lake Fazda, whereas the sediment of Lake UmRisha was dominated by members of the δ-proteobacteria. The proportion of γ-proteobacterial and Bacteroidetes-affiliated OTUs were predominant in the water of Lake UmRisha and differed significantly from other lake waters (chi-squared analysis, p ≤ 0.01). The more oxygenated and dilute nature of Lake Hamra was reflected in its microbial community composition, with the abundance of Bacillales sequences in the water, the absence of Halanaerobiales, Clostridiales, and Archaea in the water, and the presence of representatives of more phyla such as the Actinobacteria, Spirochaetes, and Verrucomicrobia. The archaeal community composition appeared less diverse: 589 clones resulted in 198 OTUs defined at 99% 16S rRNA sequence identity, and all sequences fell into the phylum Euryarchaeota. Phylogenetic analysis showed that many of the sequences were distantly related (83–90% 16S rRNA sequence identity) to cultured and uncultured archaea, with many clones forming clusters that branched deeply within the Euryarchaeota. Forty-two and 53% of the bacterial and archaeal clones had less than 90% 16S rRNA sequence identity to previously described sequences. This indicates that the water and sediments of the Wadi An Natrun harbor a unique and novel prokaryotic diversity that is different from what has been described among other alkaline, athalassohaline lakes.









Similar content being viewed by others
References
Abd-el-Malek, Y, Rizk, SG (1963) Bacterial sulfate reduction and the development of alkalinity. III. Experiments under natural conditions in the Wadi An Natrun. J Appl Microbiol 26: 20–26
Acinas, SG, Klepac-Ceraj, V, Hunt, DE, Pharino, C, Ceraj, I, Distel, DL, Polz, MF (2004) Fine-scale phylogenetic architecture of a complex bacterial community. Nature 430: 551–554
Acinas, SG, Marcelino, LA, Klepac-Ceraj, V, Polz, MF (2004) Divergence and redundancy of 16S rRNA sequences in genomes with multiple rrn operons. J Bacteriol 186: 2629–2635
Anton, J, Oren, A, Benlloch, S, Rodriguez-Valera, F, Amann, R, Rossello-Mora, R (2002) Salinibacter ruber gen. nov., sp. nov., a novel, extremely halophilic member of the Bacteria from saltern crystallizer ponds. Int J Syst Evol Microbiol 52: 485–491
Arahal, D, Garcia, M, Vargas, C, Canovas, D, Nieto, J, Ventosa, A (2001) Chromohalobacter salexigens sp. nov., a moderately halophilic species that includes Halomonas elongata DSM 3043 and ATCC 33174. Int J Syst Evol Microbiol 51: 1457–1462
Berry, A, Janssens, D, Humbelin, M, Jore, JPM, Hoste, B, Cleenwerck, I, Vancanneyt, M, Bretzel, W, Mayer, AF, Lopez-Ulibarri, R, Shanmugam, B, Swings, J, Pasamontes, L (2003) Paracoccus zeaxanthinifaciens sp. nov., a zeaxanthin-producing bacterium. Int J Syst Evol Microbiol 53: 231–238
Boone, DR (2001) Genus V. Methanolobus. In: Boone, DR, Castenholz, RW, Garrity, GM (Eds.) Bergey’s Manual of Systematic Bacteriology, 1 (The Archaea and the Deeply Branching Phototrophic Bacteria). Springer, Berlin Heidelberg New York, pp 283–287
Cao, X, Liu, X, Dong, X (2003) Alkaliphilus crotonatoxidans sp. nov., a strictly anaerobic, crotonate-dismutating bacterium isolated from a methanogenic environment. Int J Syst Evol Microbiol 53: 971–975
Demergasso, C, Casamayor, E, Chong, G, Galleguillos, P, Escudero, L, Pedros-Alio, C (2004) Distribution of prokaryotic genetic diversity in athalassohaline lakes of the Atacama Desert, Northern Chile. FEMS Microbiol Ecol 48: 57–69
Dong, H, Zhang, G, Jiang, H, Yu, B, Chapman, LR, Lucas, CR, Fields, MW (2006) Microbial diversity in sediments of saline Qinghai Lake, China: Linking geochemical controls to microbial ecology. Microb Ecol 51: 65–82
Fracek, SPJ, Stolz, JF (1985) Spirochaeta bajacaliforniensis sp. nov. from a microbial mat community at Laguna Figueroa, Baja California Norte, Mexico. Arch Microbiol 142: 317–325
Fritze, D (1996) Bacillus haloalkaliphilus sp. nov. Int J Syst Bacteriol 46: 98–101
Garcia-Pichel, F, Nübel, U, Muyzer, G (1998) The phylogeny of unicellular, extremely halotolerant cyanobacteria. Arch Microbiol 169: 469–482
Good, IJ (1953) The population frequencies of species and the estimation of population parameters. Biometrika 40: 237–264
Grant, S, Grant, WD, Jones, BE, Kato, C, Li, L (1999) Novel archaeal phylotypes from an East African alkaline saltern. Extremophiles 3: 139–145
Grant, S, Sorokin, DY, Grant, WD, Jones, BE, Heaphy, S (2004) A phylogenetic analysis of Wadi el Natrun soda lake cellulase enrichment cultures and identification of cellulase genes from these cultures. Extremophiles 8: 421–429
Grant, WD (2001) Genus I. Halobacterium. In: Boone, DR, Castenholz, RW (Eds.) Bergey’s Manual of Systematic Bacteriology, 1. Springer, Berlin Heidelberg New York, pp 301–305
Harmsen, H, Van Kuijk, B, Plugge, C, Akkermans, A, De Vos, W, Stams, A (1998) Syntrophobacter fumaroxidans sp. nov., a syntrophic propionate-degrading sulfate-reducing bacterium. Int J Syst Bacteriol 48: 1383–1387
Hirschler-Rea, A, Matheron, R, Riffaud, C, Moune, S, Eatock, C, Herbert, RA, Willison, JC, Caumette, P (2003) Isolation and characterization of spirilloid purple phototrophic bacteria forming red layers in microbial mats of Mediterranean salterns: Description of Halorhodospira neutriphila sp. nov. and emendation of the genus Halorhodospira. Int J Syst Evol Microbiol 53: 153–163
Hoover, RB, Pikuta, EV, Bej, AK, Marsic, D, Whitman, WB, Tang, J, Krader, P (2003) Spirochaeta americana sp.nov., a new haloalkaliphilic, obligately anaerobic spirochaete isolated from soda Mono Lake in California. Int J Syst Evol Microbiol 53: 815–821
Humayoun, SB, Bano, N, Hollibaugh, JT (2003) Depth distribution of microbial diversity in Mono Lake, a meromictic soda lake in California. Appl Environ Microbiol 69: 1030–1042
Imhoff, JF (2001) True marine and halophilic anoxygenic phototrophic bacteria. Arch Microbiol 176: 243–254
Imhoff, JF, Hashwa, F, Truper, HG (1978) Isolation of extremely halophilic phototrophic bacteria from the alkaline Wadi Natrun, Egypt. Arch Hydrobiol 84: 381–388
Imhoff, JF, Sahl, HG, Soliman, GS, Truper, HG (1979) The Wadi Natrun: Chemical composition and microbial mass developments in alkaline brines of eutrophic desert lakes. Geomicrobiol J 1: 219–234
Imhoff, JF, Truper, HG (1977) Ectothiorhodospira halochloris sp. nov., a new extremely halophilic phototrophic bacterium containing bacteriochlorophyll b. Arch Microbiol 114: 115–121
Jiang, H, Dong, H, Zhang, G, Yu, B, Chapman, LR, Fields, MW (2006) Microbial diversity in water and sediment of Lake Chaka, an athalassohaline lake in northwestern China. Appl Environ Microbiol 72: 3832–3845
Jones, BE, Grant, WD, Duckworth, AW, Owenson, GG (1998) Microbial diversity of soda lakes. Extremophiles 2: 191–200
Koizumi, Y, Kojima, H, Oguri, K, Kitazato, H, Fukui, M (2004) Vertical and temporal shifts in microbial communities in the water column and sediment of saline meromictic Lake Kaiike (Japan), as determined by a 16S rDNA-based analysis, and related to physicochemical gradients. Environ Microbiol 6: 622–637
Ma, Y, Zhang, W, Xue, Y, Zhou, P, Ventosa, A, Grant, WD (2004) Bacterial diversity of the Inner Mongolian Baer Soda Lake as revealed by 16S rRNA gene sequence analyses. Extremophiles 8: 45–51
Martinez-Canovas, MJ, Quesada, E, Martinez-Checa, F, Moral, Ad, Bejar, V (2004) Salipiger mucescens gen. nov., sp. nov., a moderately halophilic, exopolysaccharide-producing bacterium isolated from hypersaline soil, belonging to the α-Proteobacteria. Int J Syst Evol Microbiol 54: 1735–1740
Mathrani, IM, Boone, DR, Mah, R, Fox, GE, Lau, PP (1988) Methanohalophilus zhilinae sp. nov., an alkaliphilic, halophilic, methylotropic methanogen. Int J Syst Bacteriol 38: 139–142
Maturrano, L, Santos, F, Rossello-Mora, R, Anton, J (2006) Microbial diversity in Maras salterns, a hypersaline environment in the Peruvian Andes. Appl Environ Microbiol 72: 3887–3895
Milford, AD, Achenbach, LA, Jung, DO, Madigan, MT (2000) Rhodobaca bogoriensis gen. nov. sp. nov., an alkaliphilic purple non sulfur bacterium from African Rift Valley soda lakes. Arch Microbiol 174: 18–27
Oren, A, Rodriguez-Valera, F (2001) The contribution of halophilic Bacteria to the red coloration of saltern crystallizer ponds. FEMS Microbiol Ecol 36: 123–130
Pikuta, EV, Zhilina, TN, Zavarzin, GA, Kostrikina, NA, Osipov, GA, Rainey, FA (1998) Desulfonatronum lacustre gen. nov., sp. nov.: a new alkaliphilic sulfate-reducing bacterium utilizing ethanol. Mikrobiologiia 67: 105–113
Purdy, KJ, Cresswell-Maynard, TD, Nedewell, DB, Mcgenity, TJ, Grant, WD, Timmis, KN, Embley, TM (2004) Isolation of haloarchaea that grow at low salinities. Environ Microbiol 6: 591–595
Rainey, FA, Zhilina, TN, Boulygina, ES, Stackebrandt, E, Tourova, TP, Zavarzin, GA (1995) The taxonomic status of the fermentative halophilic anaerobic bacteria: Description of Haloanaerobiales ord. nov., Halobacteroidaceae fam. nov., Orenia gen. nov. and further taxonomic rearrangements at the genus and species level. Anaerobe 1: 185–199
Rees, HC, Grant, WD, Jones, BE, Heaphy, S (2004) Diversity of Kenyan soda lake alkaliphiles assessed by molecular methods. Extremophiles 8: 63–71
Schloss, PD, Handelsman, J (2005) Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Environ Microbiol 71: 1501–1506
Scholten, JCM, Joye, SB, Hollibaugh, JT, Murrell, JC (2005) Molecular analysis of the sulfate reducing and archaeal community in a meromictic soda lake (Mono Lake, California) by targeting 16S rRNA, mcrA, apsA, and dsrAB genes. Microb Ecol 50: 29–39
Schweinfurth, G, Lewin, L (1898) Beitrage zur topographie und geochemie des agyptischen Natron-Thals. Zeitschr Ges Erdk 33: 1–25
Singleton, DR, Furlong, MA, Rathbun, SL, Whitman, WB (2001) Quantitative comparisons of 16S rRNA gene sequence libraries from environmental samples. Appl Environ Microbiol 67: 4374–4376
Soliman, GS, Truper, HG (1982) Halobacterium pharonis sp. nov., a new, extremely haloalkaliphilic archaebacterium with low magnesium requirement. Zbl Bact Hyg I Abt Orig C 3: 318–329
Sorensen, KB, Canfield, DE, Teske, AP, Oren, A (2005) Community composition of a hypersaline endoevaporitic microbial mat. Appl Environ Microbiol 71: 7352–7365
Sorokin, DY, Gorlenko, VM, Namsaraev, BB, Namsaraev, ZB, Lysenko, AM, Eshinimaev, BT, Khmelenina, VN, Trotsenko, YA, Kuenen, JG (2004) Prokaryotic communities of north-eastern Mongolian soda lakes. Hydrobiologia 522: 235–248
Sorokin, DY, Tourova, TP, Kolganova, TV, Sjollema, KA, Kuenen, JG (2002) Thioalkalispira microaerophila gen. nov., sp. nov., a novel lithoautotrophic, sulfur-oxidizing bacterium from a soda lake. Int J Syst Evol Microbiol 52: 2175–2182
Sorokin, DY, Tourova, TP, Kuznetsov, BB, Briantseva, IA, Gorlenko, VM (2000) Roseinatronobacter thiooxidans gen. nov., sp.nov., a new alkaliphilic aerobic bacteriochlorophyll-alpha-containing bacteria from a soda lake. Mikrobiologiia 69: 89–97
Sorokin, DY, Tourova, TP, Sjollema, KA, Kuenen, JG (2003) Thialkalivibrio nitratireducens sp. nov., a nitrate-reducing member of an autotrophic denitrifying consortium from a soda lake. Int J Syst Evol Microbiol 53: 1779–1783
Taher, AG (1999) Inland saline lakes of Wadi El Natrun depression, Egypt. Int J Salt Lake Res 8: 149–170
Takai, K, Moser, D, Onstott, T, Spoelstra, N, Pfiffner, S, Dohnalkova, A, Fredrickson, J (2001) Alkaliphilus transvaalensis gen. nov., sp. nov., an extremely alkaliphilic bacterium isolated from a deep South African gold mine. Int J Syst Evol Microbiol 51: 1245–1256
Tonolla, M, Demarta, A, Peduzzi, R, Hahn, D (1999) In situ analysis of phototrophic sulfur bacteria in the chemocline of meromictic Lake Cadagno (Switzerland). Appl Environ Microbiol 65: 1325–1330
Wintzingerode, F, Gobel, UB, Stackebrandt, E (1997) Determination of microbial diversity in environmental samples: Pitfalls of PCR-based rRNA analysis. FEMS Microbiol Rev 21: 213–329
Wood, A, Kelly, D (1993) Reclassification of Thiobacillus thyasiris as Thiomicrospira thyasirae comb. nov., an organism exhibiting pleomorphism in response to environmental conditions. Arch Microbiol 159: 45–47
Yakimov, M, Giuliano, L, Chernikova, T, Gentile, G, Abraham, W, Lunsdorf, H, Timmis, K, Golyshin, P (2001) Alcalilimnicola halodurans gen. nov., sp. nov., an alkaliphilic, moderately halophilic and extremely halotolerant bacterium, isolated from sediments of soda-depositing Lake Natron, East Africa Rift Valley. Int J Syst Evol Microbiol 51: 2133–2143
Yoon, J-H, Kang, KH, Oh, T-K, Park, Y-H (2004) Erythrobacter aquimaris sp. nov., isolated from sea water of a tidal flat of the Yellow Sea in Korea. Int J Syst Evol Microbiol 54: 1981–1985
Yoon, J-H, Lee, M-H, Oh, T-K (2004) Porphyrobacter donghaensis sp. nov., isolated from sea water of the East Sea in Korea. Int J Syst Evol Microbiol 54: 2231–2235
Zhilina, TN, Appel, R, Probian, C, Brossa, EL, Harder, J, Widdel, F, Zavarzin, GA (2004) Alkaliflexus imshenetskii gen. nov. sp. nov., a new alkaliphilic gliding carbohydrate-fermenting bacterium with propionate formation from a soda lake. Arch Microbiol 182: 244–253
Acknowledgments
We would like to thank M. Mesbah for the help with sample collection at the Wadi An Natrun, J. Unrine at the Savannah River Ecology Laboratory for the help with ICP-MS analyses, and W. B. Whitman for the helpful discussion. This work was supported by NSF INT-021100 to J. Wiegel.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00248-007-9338-7
Rights and permissions
About this article
Cite this article
Mesbah, N.M., Abou-El-Ela, S.H. & Wiegel, J. Novel and Unexpected Prokaryotic Diversity in Water and Sediments of the Alkaline, Hypersaline Lakes of the Wadi An Natrun, Egypt. Microb Ecol 54, 598–617 (2007). https://doi.org/10.1007/s00248-006-9193-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-006-9193-y