Abstract
Introduction
The aim of this study was to determine the prognostic value of metabolic alterations in the normal-appearing white matter (NAWM) of patients presenting with clinically isolated syndromes (CIS) suggestive of multiple sclerosis (MS) with special regard to the prediction of conversion to definite MS.
Methods
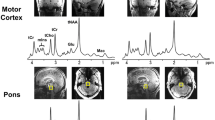
Using a 3T whole-body MR system, a multisequence conventional MRI protocol and single-voxel proton MR spectroscopy (PRESS, repetition time 2000 ms, echo times 38 ms and 140 ms) of the parietal NAWM were performed in 25 patients presenting with CIS at baseline and in 20 controls. Absolute concentrations of N-acetyl-aspartate (tNAA), myo-inositol (Ins), choline (Cho) and creatine (tCr) as well as metabolite ratios were determined. Follow-up including neurological assessment and conventional MRI was performed 3–4 and 6–7 months after the initial event.
Results
Nine patients converted to definite MS during the follow-up period. Compared to controls, those patients who converted to MS also showed significantly lower tNAA concentrations in the NAWM (−13.4%, P = 0.002) whereas nonconverters (−6.5%, P = 0.052) did not. The Ins concentration was 20.2% higher in the converter group and 1.9% higher in the nonconverter group, but these differences did not reach significance. No significant differences could be observed for tCr and Cho in either patient group.
Conclusion
Axonal damage at baseline in patients presenting with CIS was more prominent in those who subsequently converted to definite MS in the short term follow-up, indicating that tNAA might be a sufficient prognostic marker for patients with a higher risk of conversion to early definite MS.


Similar content being viewed by others
References
Rovira Canellas A, Rovira Gols A, Rio Izquierdo J, Tintoré Subirana M, Montalban Gairin X (2007) Idiopathic inflammatory-demyelinating diseases of the central nervous system. Neuroradiology 49:393–409
Miller DH, Thompson AJ, Filippi M (2003) Magnetic resonance studies of abnormalities in the normal appearing white matter and grey matter in multiple sclerosis. J Neurol 250:1407–1419
Brex PA, Ciccarelli O, O’ Riordan JI, Sailer M, Thompson AJ, Miller DH (2002) A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med 346:158–164
Barkhof F, Filippi M, Miller DH et al (1997) Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 120:2059–2069
Tintoré M, Rovira A, Martinez MJ et al (2000) Isolated demyelinating syndromes: comparison of different MR imaging criteria to predict conversion to clinically definite multiple sclerosis. AJNR Am J Neuroradiol 21:702–706
Minneboo A, Barkhof F, Polman CH et al (2004) Infratentorial lesions predict long-term disability in patients with initial findings suggestive of multiple sclerosis. Arch Neurol 61:217–221
Bitsch A, Bruhn H, Vougioukas V et al (1999) Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopy. AJNR Am J Neuroradiol 20:1619–1627
Simmons ML, Frodonza CG, Coyle JT (1991) Immunocytochemical localization of N-acetyl-aspartate with monoclonal antibodies. Neuroscience 45:37–45
Nakano M, Ueda H, Li J et al (1998) Measurement of regional N-acetylaspartate after transient global ischemia in gerbils with and without ischemic tolerance: an index of neuronal survival. Ann Neurol 44:334–340
Brand A, Richter-Landsberg C, Leibfritz D (1993) Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci 15:289–298
De Stefano N, Narayanan S, Francis GS et al (2001) Evidence of axonal damage in the early stages of multiple sclerosis and its relevance to disability. Arch Neurol 58:65–70
De Stefano N, Matthews PM, Fu L et al (1998) Axonal damage correlates with disability in patients with relapsing-remitting multiple sclerosis. Results of a longitudinal magnetic resonance spectroscopy study. Brain 121:1469–1477
Chard DT, Griffin CM, McLean MA et al (2002) Brain metabolite changes in cortical grey and normal-appearing white matter in clinically early relapsing remitting multiple sclerosis. Brain 125:2342–2352
Kapeller P, McLean MA, Griffin CM et al (2001) Preliminary evidence for neuronal damage in cortical grey and normal appearing white matter in short duration relapsing-remitting multiple sclerosis: a quantitative MR spectroscopic imaging study. J Neurol 248:131–138
Vrenken H, Barkhof F, Uitdehaag BMJ, Castelijns JA, Polman CH, Pouwels PJW (2005) MR spectroscopic evidence of glial increase but not for neuro-axonal damage in MS normal-appearing white matter. Magn Reson Med 53:256–266
Srinivasan R, Sailasuta N, Hurd R, Nelson S, Pelletier D (2005) Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3T. Brain 128:1016–1025
Tedeschi G, Bonavita S, McFarland HF, Richert N, Duyn JH, Frank JA (2002) Proton MR spectroscopic imaging in multiple sclerosis. Neuroradiology 44:37–42
Fernando KTM, McLean MA, Chard DT et al (2004) Elevated white matter myo-inositol in clinical isolated syndromes suggestive of multiple sclerosis. Brain 127:1361–1369
Polman CH, Reingold SC, Edan G et al (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 58:840–846
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33:1444–1452
Wattjes MP, Lutterbey GG, Harzheim M et al (2006) Imaging of inflammatory lesions at 3.0 Tesla in patients with clinically isolated syndromes suggestive of multiple sclerosis: a comparison of fluid-attenuated inversion recovery with T2 turbo spin-echo. Eur Radiol 16:1494–1500
Simon JH, Li D, Traboulsee A et al (2006) Standardized MR imaging protocol for multiple sclerosis: consortium of MS centers consensus guidelines. AJNR Am J Neuroradiol 27:455–461
Naressi A, Couturier C, Devos JM et al (2001) Java-based graphical user interface for the MRUI quantitation package. MAGMA 12:141–152
Vanhamme L, van den Boogaart A, van Huffel S (1997) Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 129:35–43
Wattjes MP, Harzheim M, Lutterbey GG, Klotz L, Schild HH, Träber F (2007) Axonal damage but no increased glial cell activity in the normal-appearing white matter of patients with clinically isolated syndromes suggestive of multiple sclerosis using high field magnetic resonance spectroscopy. AJNR Am J Neuroradiol 28:1517–1522
Fernando KTM, Tozer DJ, Mizkiel KA et al (2005) Magnetization transfer histograms in clinically isolated syndromes suggestive of multiple sclerosis. Brain 128:2911–2925
Vrenken H, Rombouts SARB, Pouwels PJW, Barkhof F (2006) Voxel -based analysis of quantitative T1 maps demonstrates that multiple sclerosis acts throughout the normal-appearing white matter. AJNR Am J Neuroradiol 27:868–874
Vrenken H, Pouwels PJW, Geurts JJG et al (2006) Altered diffusion tensor in multiple sclerosis normal-appearing brain tissue: cortical diffusion seem related to clinical deterioration. J Magn Reson Imaging 23:628–636
Nielsen JM, Korteweg T, Barkhof F, Uitdehaag BMJ, Polman CH (2005) Overdiagnosis of multiple sclerosis and magnetic resonance imaging criteria. Ann Neurol 58:781–783
Wattjes MP, Lutterbey GG, Harzheim M et al (2006) Higher sensitivity in the detection of inflammatory brain lesions in patients with clinically isolated syndromes suggestive of multiple sclerosis using high field MRI: an intraindividual comparison of 1.5T with 3.0T. Eur Radiol 16:2067–2073
Keiper MD, Grossmann RI, Hirsch JA et al (1998) MR identification of white matter abnormalities in multiple sclerosis: a comparison between 1.5T and 4T. AJNR Am J Neuroradiol 19:1489–1493
Wattjes MP, Harzheim M, Kuhl CK et al (2006) Does high-field MRI have an influence on the classification of patients with clinically isolated syndromes according to current diagnostic magnetic resonance imaging criteria for multiple sclerosis? AJNR Am J Neuroradiol 27:1794–1798
Träber F, Block W, Lamerichs R, Gieseke J, Schild HH (2004) 1H metabolite relaxation times at 3.0 Tesla: measurements of T1 and T2 values in normal brain and determination of regional differences in transverse relaxation. J Magn Reson Imaging 19:537–545
Srinivasan R, Vigneron D, Sailasuta N, Hurd R, Nelson S (2004) A comparative study of myoinositol quantification using LCmodel at 1.5 T and 3.0 T with 3 D 1H proton spectroscopic imaging of the human brain. Magn Reson Imaging 22:523–528
Tourbah A, Stievenart JL, Abanou A et al (1999) Normal-appearing white matter in optic neuritis and multiple sclerosis: a comparative proton spectroscopy study. Neuroradiology 41:738–743
Fernando KTM, Chard DT, McLean MA et al (2006) Metabolite changes in the white matter 3 years after a clinically isolated syndrome suggestive of multiple sclerosis. Mult Scler 12(S1):41
Pit D, Werner P, Raine C (2000) Glutamate excitotoxicity in a model of multiple sclerosis. Nat Med 6:67–70
Cianfoni A, Niku S, Imbesi SG (2007) Metabolite findings in tumefactive demyelinating lesions utilizing short echo time proton magnetic resonance spectroscopy. AJNR Am J Neuroradiol 28:272–277
Acknowledgements
The authors would like to thank all patients and control subjects who took part in this study. MPW was supported by the European Exchange Program provided by the European Society of Neuroradiology (ESNR).
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest statement
We declare that we have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wattjes, M.P., Harzheim, M., Lutterbey, G.G. et al. Prognostic value of high-field proton magnetic resonance spectroscopy in patients presenting with clinically isolated syndromes suggestive of multiple sclerosis. Neuroradiology 50, 123–129 (2008). https://doi.org/10.1007/s00234-007-0325-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-007-0325-y