Abstract
Objective
To study the effects of CYP2C9 and CYP2C19 genetic polymorphisms on the pharmacokinetics and pharmacodynamics of glipizide.
Methods
Eighteen healthy male subjects were divided into three groups according to their genotypes: group I, CYP2C9*1/*1 and CYP2C19 extensive metabolizers (EMs); group II, CYP2C9*1/*1 and CYP2C19 poor metabolizers (PMs); and group III, CYP2C9*1/*3 and CYP2C19 EMs. After a single dose of a 5-mg glipizide tablet, plasma concentrations of glipizide for a 36-h period were determined. Meanwhile, plasma glucose levels and plasma insulin levels were determined from 0 to 4 h after dosing.
Results
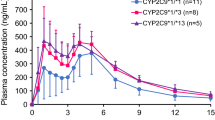
The area under the plasma concentration-time curve (\( AU{C_{0 - \infty }} \)) was 2.0-fold higher and the oral clearance was 51.1% lower in group III than in group I. The change in fasting insulin level within 1 h (ΔAUECinsulin0–1h) in group III was 3.8-fold higher than that in group I. The glipizide parameters in group II exhibited similar tendencies to those in group III.
Conclusions
These results suggest that CYP2C9 polymorphism significantly influences the pharmacokinetics and pharmacodynamics of glipizide, which needs to be considered in clinical practice. CYP2C19 polymorphism exhibits a tendency to influence the effects of glipizide, to a certain extent similarly to CYP2C9 polymorphism.




Similar content being viewed by others
References
Fuccella LM, Tamassia V, Valzelli G (1973) Metabolism and kinetics of the hypoglycemic agent glipizide in man — comparison with glibenclamide. J Clin Pharmacol New Drug 13:68–75
Balant L, Fabre J, Zahnd GR (1975) Comparison of the pharmacokinetics of glipizide and glibenclamide in man. Eur J Clin Pharmacol 8:63–69
Kidd RS, Straughn AB, Meyer MC, Blaisdell J, Goldstein JA, Dalton JT (1999) Pharmacokinetics of chlorpheniramine, phenytoin, glipizide and nifedipine in an individual homozygous for the CYP2C9*3 allele. Pharmacogenetics 9:71–80
Bae JW, Kim NT, Choi CI, Kim MJ, Jang CG, Lee SY (2007) Effects of CYP2C9 genetic polymorphism on the pharmacokinetics and pharmacodynamics of glipizide in healthy Korean subjects. FASEB J 21:lb362
Desta Z, Zhao X, Shin JG, Flockhart DA (2002) Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet 41:913–958
Wang SL, Huang J, Lai MD, Tsai JJ (1995) Detection of CYP2C9 polymorphism based on the polymerase chain reaction in Chinese. Pharmacogenetics 5:37–42
De Morais SMF, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA, Goldstein JA (1994) The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem 269:15419–15422
De Morais SMF, Wilkinson GR, Blaisdell J, Meyer UA, Nakamura K, Goldstein JA (1994) Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol 46:594–598
Zhao XH, Song B, Zhong DF, Zhang SQ, Chen XY (2007) Simultaneous determination of metformin and glipizide in human plasma by liquid chromatography-tandem mass spectrometry. Acta Pharm Sin 42:1087–1091
Park JY, Kim KA, Park PW, Park CW, Shin JG (2003) Effect of rifampin on the pharmacokinetics and pharmacodynamics of gliclazide. Clin Pharmacol Ther 74:334–340
Xie HG, Prasad HC, Kim RB, Stein CM (2002) CYP2C9 allelic variants: ethnic distribution and functional significance. Adv Drug Deliv Rev 54:1257–1270
Lee CR, Goldstein JA, Pieper JA (2002) Cytochrome P450 2C9 polymorphisms: a comprehensive review of the in-vitro and human data. Pharmacogenetics 12:251–263
Sim SC, Risinger C, Dahl ML, Aklillu E, Christensen M, Bertilsson L et al (2006) A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther 79:103–113
Wang G, Lei HP, Li Z, Tan ZR, Guo D, Fan L et al (2009) The CYP2C19 ultra-rapid metabolizer genotype influences the pharmacokinetics of voriconazole in healthy male volunteers. Eur J Clin Pharmacol 65:281–285
Cefalu WT (2007) Pharmacotherapy for the treatment of patients with type 2 diabetes mellitus: rationale and specific agents. Clin Pharmacol Ther 81:636–649
Lindh JD, Holm L, Andersson ML, Rane A (2009) Influence of CYP2C9 genotype on warfarin dose requirements—a systematic review and meta-analysis. Eur J Clin Pharmacol 65:365–375
Becquemont L (2008) Evidence for a pharmacogenetic adapted dose of oral anticoagulant in routine medical practice. Eur J Clin Pharmacol 64:953–960
Acknowledgments
This study was supported by the Natural Science Foundation of China grant 30772621.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, B., Zhang, YF., Chen, XY. et al. The effects of CYP2C9 and CYP2C19 genetic polymorphisms on the pharmacokinetics and pharmacodynamics of glipizide in Chinese subjects. Eur J Clin Pharmacol 66, 145–151 (2010). https://doi.org/10.1007/s00228-009-0736-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-009-0736-2