Abstract
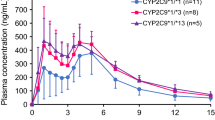
Gliclazide metabolism is mediated by genetically polymorphic CYP2C9 and CYP2C19 enzymes. We investigated the effects of CYP2C9 and CYP2C19 genetic polymorphisms on the pharmacokinetics and pharmacodynamics of gliclazide. Twenty-seven Korean healthy volunteers were administered a single oral dose of gliclazide 80 mg. The plasma concentration of gliclazide was quantified for the pharmacokinetic analysis and plasma concentrations of glucose and insulin were measured as pharmacodynamic parameters. The pharmacokinetics of gliclazide showed a significant difference according to the number of defective alleles of combined CYP2C9 and CYP2C19. The two defective alleles group (group 3) and one defective allele group (group 2) showed 2.34- and 1.46-fold higher AUC0–∞ (P < 0.001), and 57.1 and 32.3% lower CL/F (P < 0.001), compared to those of the no defective allele group (group 1), respectively. The CYP2C9IM–CYP2C19IM group had AUC0–∞ increase of 1.49-fold (P < 0.05) and CL/F decrease by 29.9% (P < 0.01), compared with the CYP2C9 Normal Metabolizer (CYP2C9NM)–CYP2C19IM group. The CYP2C9NM–CYP2C19PM group and CYP2C9NM–CYP2C19IM group showed 2.41- and 1.51-fold higher AUC0–∞ (P < 0.001), and 59.6 and 35.4% lower CL/F (P < 0.001), compared to those of the CYP2C9NM–CYP2C19NM group, respectively. The results represented that CYP2C9 and CYP2C19 genetic polymorphisms significantly affected the pharmacokinetics of gliclazide. Although the genetic polymorphism of CYP2C19 had a greater effect on the pharmacokinetics of gliclazide, the genetic polymorphism of CYP2C9 also had a significant effect. On the other hand, plasma glucose and insulin responses to gliclazide were not significantly affected by the CYP2C9–CYP2C19 genotypes, requiring further well-controlled studies with long-term dosing of gliclazide in diabetic patients.






Similar content being viewed by others
References
Bae JW, Kim HK, Kim JH, Yang SI, Kim MJ, Jang CG, Park YS, Lee SY (2005) Allele and genotype frequencies of CYP2C9 in a korean population. Br J Clin Pharmacol 60(4):418–422. https://doi.org/10.1111/j.1365-2125.2005.02448.x
Bae JW, Choi CI, Jang CG, Lee SY (2011a) Effects of CYP2C9*1/*13 on the pharmacokinetics and pharmacodynamics of meloxicam. Br J Clin Pharmacol 71(4):550–555. https://doi.org/10.1111/j.1365-2125.2010.03853.x
Bae JW, Choi CI, Kim MJ, Oh DH, Keum SK, Park JI, Kim BH, Bang HK, Oh SG, Kang BS, Park HJ, Kim HD, Ha JH, Shin HJ, Kim YH, Na HS, Chung MW, Jang CG, Lee SY (2011) Frequency of CYP2C9 alleles in koreans and their effects on losartan pharmacokinetics. Acta Pharmacol Sin 32(10):1303–1308. https://doi.org/10.1038/aps.2011.100
Bae JW, Choi CI, Lee HI, Lee YJ, Jang CG, Lee SY (2012) Effects of CYP2C9*1/*3 and *1/*13 on the pharmacokinetics of losartan and its active metabolite E-3174. Int J Clin Pharmacol Ther 50(9):683–689. https://doi.org/10.5414/cp201467
Bae JW, Oh KY, Yoon SJ, Shin HB, Jung EH, Cho CK, Lim CW, Kang P, Choi CI, Jang CG, Lee SY, Lee YJ (2020) Effects of CYP2D6 genetic polymorphism on the pharmacokinetics of metoclopramide. Arch Pharm Res 43(11):1207–1213. https://doi.org/10.1007/s12272-020-01293-4
Caudle KE, Dunnenberger HM, Freimuth RR, Peterson JF, Burlison JD, Whirl-Carrillo M, Scott SA, Rehm HL, Williams MS, Klein TE, Relling MV, Hoffman JM (2017) Standardizing terms for clinical pharmacogenetic test results: consensus terms from the clinical pharmacogenetics implementation consortium (CPIC). Genet Med 19(2):215–223. https://doi.org/10.1038/gim.2016.87
Chen Y, Chen L, Zhang H, Huang S, Xiong Y, Xia C (2018) Interaction of sulfonylureas with liver uptake transporters OATP1B1 and OATP1B3. Basic Clin Pharmacol Toxicol 123(2):147–154. https://doi.org/10.1111/bcpt.12992
Cho CK, Kang P, Park HJ, Lee YJ, Bae JW, Jang CG, Lee SY (2021a) Physiologically based pharmacokinetic (PBPK) modelling of tamsulosin related to CYP2D6*10 allele. Arch Pharm Res 44(11):1037–1049. https://doi.org/10.1007/s12272-021-01357-z
Cho CK, Park HJ, Kang P, Moon S, Lee YJ, Bae JW, Jang CG, Lee SY (2021b) Physiologically based pharmacokinetic (PBPK) modeling of meloxicam in different CYP2C9 genotypes. Arch Pharm Res 44(12):1076–1090. https://doi.org/10.1007/s12272-021-01361-3
Cho CK, Kang P, Park HJ, Ko E, Mu CY, Lee YJ, Choi CI, Kim HS, Jang CG, Bae JW, Lee SY (2022) Physiologically based pharmacokinetic (PBPK) modeling of piroxicam with regard to CYP2C9 genetic polymorphism. Arch Pharm Res 45(5):352–366. https://doi.org/10.1007/s12272-022-01388-0
Cho CK, Byeon JY, Kang P, Park HJ, Ko E, Mu CY, Jang CG, Lee SY, Lee YJ (2023a) Effects of CYP2C19 genetic polymorphism on the pharmacokinetics of tolperisone in healthy subjects. Arch Pharm Res 46(2):111–116. https://doi.org/10.1007/s12272-022-01423-0
Cho CK, Byeon JY, Kang P, Park JI, Jang CG, Lee SY, Choi CI, Bae JW, Lee YJ (2023b) Effects of CYP2D6*10 allele on the pharmacokinetics of tolperisone. Arch Pharm Res 46(1):59–64. https://doi.org/10.1007/s12272-022-01422-1
Choi CI, Kim MJ, Jang CG, Park YS, Bae JW, Lee SY (2011) Effects of the CYP2C9*1/*13 genotype on the pharmacokinetics of lornoxicam. Basic Clin Pharmacol Toxicol 109(6):476–480. https://doi.org/10.1111/j.1742-7843.2011.00751.x
Choi CI, Kim MJ, Chung EK, Lee HI, Jang CG, Bae JW, Lee SY (2012) CYP2C9*3 and *13 alleles significantly affect the pharmacokinetics of irbesartan in healthy korean subjects. Eur J Clin Pharmacol 68(2):149–154. https://doi.org/10.1007/s00228-011-1098-0
Choi CI, Bae JW, Lee YJ, Lee HI, Jang CG, Lee SY (2014) Effects of CYP2C19 genetic polymorphisms on atomoxetine pharmacokinetics. J Clin Psychopharmacol 34(1):139–142. https://doi.org/10.1097/JCP.0b013e3182a608a2
Chow E, Poon EW, Fok BS, Chan JC, Tomlinson B (2019) CYP2C19*2 polymorphism is associated with impaired oral clearance of gliclazide in healthy chinese. Pharmgenomics Pers Med 12:397–401. https://doi.org/10.2147/PGPM.S226200
Dawed AY, Yee SW, Zhou K, van Leeuwen N, Zhang Y, Siddiqui MK, Etheridge A, Innocenti F, Xu F, Li JH, Beulens JW, van der Heijden AA, Slieker RC, Chang YC, Mercader JM, Kaur V, Witte JS, Lee MTM, Kamatani Y, Momozawa Y, Kubo M, Palmer CNA, Florez JC, Hedderson MM, ’t Hart LM, Giacomini KM, Pearson ER (2021) Genome-wide Meta-analysis identifies genetic Variants Associated with Glycemic Response to Sulfonylureas. Diabetes Care 44(12):2673–2682. https://doi.org/10.2337/dc21-1152
Elliot DJ, Suharjono, Lewis BC, Gillam EM, Birkett DJ, Gross AS, Miners JO (2007) Identification of the human cytochromes P450 catalysing the rate-limiting pathways of gliclazide elimination. Br J Clin Pharmacol 64(4):450–457. https://doi.org/10.1111/j.1365-2125.2007.02943.x
Foroutan SM, Zarghi A, Shafaati A, Khoddam A (2006) Application of monolithic column in quantification of gliclazide in human plasma by liquid chromatography. J Pharm Biomed Anal 42(4):513–516. https://doi.org/10.1016/j.jpba.2006.05.003
Gökalp O, Gunes A, Cam H, Cure E, Aydın O, Tamer MN, Scordo MG, Dahl ML (2011) Mild hypoglycaemic attacks induced by sulphonylureas related to CYP2C9, CYP2C19 and CYP2C8 polymorphisms in routine clinical setting. Eur J Clin Pharmacol 67(12):1223–1229. https://doi.org/10.1007/s00228-011-1078-4
Holstein A, Plaschke A, Ptak M, Egberts EH, El-Din J, Brockmöller J, Kirchheiner J (2005) Association between CYP2C9 slow metabolizer genotypes and severe hypoglycaemia on medication with sulphonylurea hypoglycaemic agents. Br J Clin Pharmacol 60(1):103–106. https://doi.org/10.1111/j.1365-2125.2005.02379.x
Jung EH, Cho CK, Kang P, Park HJ, Lee YJ, Bae JW, Choi CI, Jang CG, Lee SY (2021) Physiologically based pharmacokinetic modeling of candesartan related to CYP2C9 genetic polymorphism in adult and pediatric patients. Arch Pharm Res 44(12):1109–1119. https://doi.org/10.1007/s12272-021-01363-1
Kim SH, Kim DH, Byeon JY, Kim YH, Kim DH, Lim HJ, Lee CM, Whang SS, Choi CI, Bae JW, Lee YJ, Jang CG, Lee SY (2017) Effects of CYP2C9 genetic polymorphisms on the pharmacokinetics of celecoxib and its carboxylic acid metabolite. Arch Pharm Res 40(3):382–390. https://doi.org/10.1007/s12272-016-0861-2
Kim YH, Kang P, Cho CK, Jung EH, Park HJ, Lee YJ, Bae JW, Jang CG, Lee SY (2021) Physiologically based pharmacokinetic (PBPK) modeling for prediction of celecoxib pharmacokinetics according to CYP2C9 genetic polymorphism. Arch Pharm Res 44(7):713–724. https://doi.org/10.1007/s12272-021-01346-2
Kim NT, Cho CK, Kang P, Park HJ, Lee YJ, Bae JW, Jang CG, Lee SY (2022) Effects of CYP2C9*3 and *13 alleles on the pharmacokinetics and pharmacodynamics of glipizide in healthy korean subjects. Arch Pharm Res 45(2):114–121. https://doi.org/10.1007/s12272-021-01366-y
Kirchheiner J, Brockmöller J, Meineke I, Bauer S, Rohde W, Meisel C, Roots I (2002) Impact of CYP2C9 amino acid polymorphisms on glyburide kinetics and on the insulin and glucose response in healthy volunteers. Clin Pharmacol Ther 71(4):286–296. https://doi.org/10.1067/mcp.2002.122476
Kirchheiner J, Roots I, Goldammer M, Rosenkranz B, Brockmöller J (2005) Effect of genetic polymorphisms in cytochrome p450 (CYP) 2C9 and CYP2C8 on the pharmacokinetics of oral antidiabetic drugs: clinical relevance. Clin Pharmacokinet 44(12):1209–1225. https://doi.org/10.2165/00003088-200544120-00002
Klen J, Dolžan V, Janež A (2014) CYP2C9, KCNJ11 and ABCC8 polymorphisms and the response to sulphonylurea treatment in type 2 diabetes patients. Eur J Clin Pharmacol 70(4):421–428. https://doi.org/10.1007/s00228-014-1641-x
Lee YJ, Byeon JY, Kim YH, Kim SH, Choi CI, Bae JW, Sohn UD, Jang CG, Lee J, Lee SY (2015) Effects of CYP2C9*1/*3 genotype on the pharmacokinetics of flurbiprofen in korean subjects. Arch Pharm Res 38(6):1232–1237. https://doi.org/10.1007/s12272-015-0580-0
Lee HI, Byeon JY, Kim YH, Lee CM, Choi CI, Jang CG, Bae JW, Lee YJ, Lee SY (2018) Effects of CYP2C19 and CYP3A5 genetic polymorphisms on the pharmacokinetics of cilostazol and its active metabolites. Eur J Clin Pharmacol 74(11):1417–1426. https://doi.org/10.1007/s00228-018-2522-5
Lee CM, Kang P, Cho CK, Park HJ, Lee YJ, Bae JW, Choi CI, Kim HS, Jang CG, Lee SY (2022) Physiologically based pharmacokinetic modelling to predict the pharmacokinetics of metoprolol in different CYP2D6 genotypes. Arch Pharm Res 45(6):433–445. https://doi.org/10.1007/s12272-022-01394-2
Li-Wan-Po A, Girard T, Farndon P, Cooley C, Lithgow J (2010) Pharmacogenetics of CYP2C19: functional and clinical implications of a new variant CYP2C19*17. Br J Clin Pharmacol 69(3):222–230. https://doi.org/10.1111/j.1365-2125.2009.03578.x
Niemi M, Cascorbi I, Timm R, Kroemer HK, Neuvonen PJ, Kivistö KT (2002) Glyburide and glimepiride pharmacokinetics in subjects with different CYP2C9 genotypes. Clin Pharmacol Ther 72(3):326–332. https://doi.org/10.1067/mcp.2002.127495
Ragia G, Petridis I, Tavridou A, Christakidis D, Manolopoulos VG (2009) Presence of CYP2C9*3 allele increases risk for hypoglycemia in type 2 diabetic patients treated with sulfonylureas. Pharmacogenomics 10(11):1781–1787. https://doi.org/10.2217/pgs.09.96
Ragia G, Atzemian N, Maslarinou A, Manolopoulos VG (2022) SLCO1B1 c.521T>C gene polymorphism decreases hypoglycemia risk in sulfonylurea-treated type 2 diabetic patients. Drug Metab Pers Ther 37(4):347–352. https://doi.org/10.1515/dmpt-2022-0131
Rieutord A, Stupans I, Shenfield GM, Gross AS (1995) Gliclazide hydroxylation by rat liver microsomes. Xenobiotica 25(12):1345–1354. https://doi.org/10.3109/00498259509061922
Scott SA, Sangkuhl K, Gardner EE, Stein CM, Hulot JS, Johnson JA, Roden DM, Klein TE, Shuldiner AR (2011) Clinical pharmacogenetics implementation consortium guidelines for cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy. Clin Pharmacol Ther 90(2):328–332. https://doi.org/10.1038/clpt.2011.132
Shao H, Ren XM, Liu NF, Chen GM, Li WL, Zhai ZH, Wang DW (2010) Influence of CYP2C9 and CYP2C19 genetic polymorphisms on pharmacokinetics and pharmacodynamics of gliclazide in healthy chinese Han volunteers. J Clin Pharm Ther 35(3):351–360. https://doi.org/10.1111/j.1365-2710.2009.01134.x
Shin HB, Jung EH, Kang P, Lim CW, Oh KY, Cho CK, Lee YJ, Choi CI, Jang CG, Lee SY, Bae JW (2020) ABCB1 c.2677G>T/c.3435 C>T diplotype increases the early-phase oral absorption of losartan. Arch Pharm Res 43(11):1187–1196. https://doi.org/10.1007/s12272-020-01294-3
Sim SC, Risinger C, Dahl ML, Aklillu E, Christensen M, Bertilsson L, Ingelman-Sundberg M (2006) A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther 79(1):103–113. https://doi.org/10.1016/j.clpt.2005.10.002
Suzuki K, Yanagawa T, Shibasaki T, Kaniwa N, Hasegawa R, Tohkin M (2006) Effect of CYP2C9 genetic polymorphisms on the efficacy and pharmacokinetics of glimepiride in subjects with type 2 diabetes. Diabetes Res Clin Pract 72(2):148–154. https://doi.org/10.1016/j.diabres.2005.09.019
Whang SS, Cho CK, Jung EH, Kang P, Park HJ, Lee YJ, Choi CI, Bae JW, Kim HS, Jang CG, Lee SY (2022) Physiologically based pharmacokinetic (PBPK) modeling of flurbiprofen in different CYP2C9 genotypes. Arch Pharm Res 45(8):584–595. https://doi.org/10.1007/s12272-022-01403-4
Xu H, Williams KM, Liauw WS, Murray M, Day RO, McLachlan AJ (2008) Effects of St John’s wort and CYP2C9 genotype on the pharmacokinetics and pharmacodynamics of gliclazide. Br J Pharmacol 153(7):1579–1586. https://doi.org/10.1038/sj.bjp.0707685
Yang F, Xiong X, Liu Y, Zhang H, Huang S, Xiong Y, Hu X, Xia C (2018) CYP2C9 and OATP1B1 genetic polymorphisms affect the metabolism and transport of glimepiride and gliclazide. Sci Rep 8(1):10994. https://doi.org/10.1038/s41598-018-29351-4
Yin OQ, Tomlinson B, Chow MS (2005) CYP2C9, but not CYP2C19, polymorphisms affect the pharmacokinetics and pharmacodynamics of glyburide in chinese subjects. Clin Pharmacol Ther 78(4):370–377. https://doi.org/10.1016/j.clpt.2005.06.006
Zeng W, Guo Y, Chen P, Liu Z, Chen D, Han C (2016) CYP2C9*3 variant is associated with antidiabetes efficacy of gliclazide in chinese type 2 diabetes patients. J Diabetes Investig 7(5):764–768. https://doi.org/10.1111/jdi.12486
Zhang Y, Si D, Chen X, Lin N, Guo Y, Zhou H, Zhong D (2007) Influence of CYP2C9 and CYP2C19 genetic polymorphisms on pharmacokinetics of gliclazide MR in chinese subjects. Br J Clin Pharmacol 64(1):67–74. https://doi.org/10.1111/j.1365-2125.2007.02846.x
Zhou SF, Liu JP, Chowbay B (2009) Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev 41(2):89–295. https://doi.org/10.1080/03602530902843483
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2019R1A2C1004582) and a grant from the Ministry of Food and Drug Safety.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, P., Cho, CK., Jang, CG. et al. Effects of CYP2C9 and CYP2C19 genetic polymorphisms on the pharmacokinetics and pharmacodynamics of gliclazide in healthy subjects. Arch. Pharm. Res. 46, 438–447 (2023). https://doi.org/10.1007/s12272-023-01448-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-023-01448-z